Cataract-refractive surgery has become increasingly demanding as patient expectations have risen. Technology has responded with improved preoperative assessment and testing, and the advent of new-generation IOL power formulas has also contributed to better accuracy. Surgeons are getting closer to hitting their targets consistently, as evidenced by several studies. For instance, 44.6 to 58.4 percent of patients achieved a ±0.5 D deviation between 1996 and 2005, a proportion that increased to between 61.2 and 88 percent between 2007 and 2017.1
“I think the impetus to improve our refractive results has been stimulated by the increased use of premium lens implants that has occurred over time,” says Jonathan B. Rubenstein, MD, chairman of the department of ophthalmology at Rush University Medical Center in Chicago. “Now that patients have options for paying out of pocket for a more premium type of lens implant product, the bar goes up to achieve satisfaction. If they’re paying something out of pocket, they want to be satisfied with the outcome. There’s greater pressure on making sure that we get accurate preoperative results when assessing and measuring these patients so that we get accurate postoperative refractions afterwards.”
Despite this progress, a percentage of post-cataract patients will experience some postoperative glare, halos and starburst symptoms.1 This could be due to a number of factors, including inaccurate preoperative biometry, ocular comorbidities, a history of laser vision correction and dry-eye disease, among others. We spoke with cataract-refractive surgeons about how they approach these patients, their strategies for determining the root cause of the issue(s) and what influences their treatment decision.
 |
|
If conditions such as epithelial basement membrane dystrophy or punctate keratitis are missed during preoperative screening, then the patient’s biometry, topography and IOL calculations may be affected. Photo: Alice T. Epitropoulos, MD. |
Getting to the Cause
One of the best strategies for dealing with unhappy patients is to address their expectations in advance, say surgeons. Premium lenses have made this preoperative conversation even more important.
Alice T. Epitropoulos, MD, FACS, who practices in Ohio and is a clinical assistant professor at The Ohio State University, says she tells patients there’s no perfect lens. “I emphasize that there isn’t a one-size-fits-all solution; some compromises may be necessary,” she says. “For instance, while extended depth of focus lenses provide a broad range of vision, these implants prioritize distance and intermediate vision, however, near vision may not be as sharp as with a multifocal or trifocal implant. Trifocal implants provide clear vision at near, intermediate and distance ranges, but may be associated with glare or halos at night.”
The ocular surface must also be pristine, she continues. “Premium lenses require more intensive treatment for dry-eye disease, just to ensure accurate biometry and to minimize the higher order aberrations. These patients really do have a greater sensitivity to ocular surface irregularities, especially when it comes to multifocal technology. A dysfunctional ocular surface is a very frequent cause of patient dissatisfaction postoperatively. In fact, dry-eye disease is a common reason why patients are poor candidates for presbyopia-correcting technology initially,” Dr. Epitropoulos says. “Taking that extra chair time with patients really helps to prevent surprises and disappointment in patients. This is particularly important in patients who’ve had previous laser vision correction and have expressed an interest in premium lens technology. The presence of prior refractive surgery adds complexity to IOL calculations and can induce higher-order aberrations. Despite the potential for achieving complete spectacle independence, it’s important never to guarantee this to patients.”
A refractive miss can be identified within a week after surgery, but surgeons shouldn’t jump right into fixing it.
“If a patient is unhappy with their surgical outcome, it’s essential to investigate the underlying reasons,” says Dr. Epitropoulos. “Could it be due to untreated or inadequately treated dry-eye disease? If so, aggressive treatment of their dry eye (preferably preoperatively), often leads to satisfaction. However, if preoperative measurements such as IOLMaster or topography were inaccurate preoperatively, this may lead to residual refractive errors, which may need to be addressed. Typically, I prefer to wait three or four months, allowing for neuroadaptation before considering invasive interventions, such as IOL exchange or laser vision correction. Oftentimes, the brain can adjust to the new optical system, avoiding additional procedures.”
Surgeons should turn to their preop diagnostic tools to accurately assess the situation, recommends John Berdahl, MD, a partner at Vance Thompson Vision in Sioux Falls, South Dakota. “The first thing that you need to do is a good refraction to determine if it is refractive error in the first place,” he says. “A second important diagnostic is topography. A third important diagnostic is an OCT of the macula, a fourth important diagnostic that’s widely underutilized is epithelial mapping. That can help show us subtle anterior basement membrane dystrophy or irregular epithelium, and then the fifth is a gas-permeable over-refraction to help us determine if the anterior surface of the cornea is the source of error for the vision. Of course, we’re looking for all of the other potential comorbidities: glaucoma; macular degeneration; epiretinal membrane; subtle cystoid macular edema; and we’d target our therapies at those approaches.”
Dr. Berdahl says surgically induced astigmatism accounts for a surprising amount of refractive misses. “The standard deviation around surgically induced astigmatism is much higher than we generally think that it is and it’s a little bit difficult to predict postoperative surgically induced astigmatism,” he explains.
He also evaluated if multifocal toric IOLs left patients worse off than monofocal toric options. “Interestingly, we looked at data from Astigmatismfix.com and the impact of residual cylinder on visual acuity and found that monofocals didn’t have worse visual acuity than multifocals for the same level of residual astigmatism,” says Dr. Berdahl. “One thing our study couldn’t assess of course is visual quality, which may or may not be decreased in patients who have residual refractive error in multifocality.”2
 |
|
Post-cataract refractive errors are typically low and surgeons say limbal-relaxing incisions are a useful technique for correcting up to 2 D of astigmatism. Photo: Jonathan B. Rubenstein, MD. |
Handling the Ocular Surface
If a contributing culprit is determined to be some form of dry-eye disease, it should be treated accordingly before even considering other interventions.
Ideally, dry-eye disease would be caught preoperatively. Dr. Epitropoulos says she abides by the oft-repeated quote by Eric Donnenfeld, MD: “If you diagnose dry eye preoperatively, it’s an expectation, if you don’t diagnose until after surgery, it’s a complication.”
“Many people aren’t aware of having dry-eye disease until they start experiencing symptoms after surgery, leading them to attribute their discomfort to the procedure,” she says. “Whereas, if asymptomatic dry-eye is identified before surgery and patients are informed that they may experience dry-eye symptoms afterwards, they are more prepared for it. Prevention, in this case, is the most effective approach to managing this issue.
“If by chance it goes undetected preoperatively, the goal is to identify the type and severity of their dry-eye disease or their ocular surface disease,” continues Dr. Epitropoulos. “Is it meibomian gland dysfunction or aqueous deficient dry-eye disease? Or is it a combination? Is there a possibility of another contributing factor such as anterior basement membrane disease, that may have been overlooked preop?”
One of the first-line treatments she recommends is a high-quality, re-esterified Omega 3 as a fundamental component in managing DED.
A preservative-free, artificial tear is another consideration. “Avoiding preservatives is important to prevent toxicity to the cornea,” says Dr. Epitropoulos. “When recommending an artificial tear, opting for those with added oil or lipid is beneficial, especially in addressing MGD or evaporative dry-eye disease that we commonly see. My threshold for prescribing an immunomodulator is relatively low because many patients have an underlying inflammatory component to their dry eye. Prescribing an immunomodulator helps to increase that tear production and reduces inflammation. It’s essential to allow sufficient time for these medications to take effect as they may not yield immediate results. There are several effective options available, including cyclosporines, Lifitegrast and Miebo.”
Many cataract surgeons have adopted in-office dry-eye treatments, including LipiFlow, iLux, TearCare and intense pulsed light therapy. “These can be performed preferably before surgery, but can also be considered postoperatively if patients are symptomatic and having problems with DED,” continues Dr. Epitropoulos. “Typically, I avoid performing these procedures immediately after surgery to prevent any interference with the wound or incision. Instead, I wait for several weeks before considering such interventions. Additionally, I incorporate amniotic membrane therapy into my treatment approach, particularly for patients that have moderate to severe dry-eye disease. This therapy can also be performed pre- or postoperatively.”
Corneal-Based Refractive Procedures
Once the ocular surface has been addressed or ruled out, patients have a few options for moving forward with correcting their error, including laser correction with LASIK or PRK, or astigmatic incisions, depending on the degree of error.
Dr. Berdahl says it’s common and helpful to give the patient glasses in the early postop period to determine if the refractive error is the problem. “We have them wear a temporary pair of glasses until they’re three months out from their surgery before considering options,” he says. “It also serves to decrease the anxiety level of the patient because they know that they can see okay, and they know the problem is fixable. Imagine you had cataract surgery, you’ve got 1 D of cylinder, you don’t like your vision and your doctor says: ‘Don’t worry, it’ll be fine. Just live with the blurry vision for three months.’ No, let’s give them a temporary pair of glasses and make them see better.”
However, if patients are adamant about not wearing glasses, then PRK or LASIK is an option.
“If it’s astigmatism only and their spherical equivalent is near plano or zero, then astigmatic incisions work extremely well and allow you to correct astigmatism, without altering their spherical power,” says Dr. Rubenstein. “If they have a combination of myopia and astigmatism or hyperopia and astigmatism, then you might want to consider either PRK or LASIK. The decision of which of those two procedures to perform depends on the patient’s ocular surface and how you think they’re going to heal.”
Surgeons differ on their preferred method for the corneal refractive procedure, and take patient-specific factors into account.
“If they’re very dry, then LASIK may not be a good procedure and PRK may be better,” Dr. Rubenstein says. “If they actually have true corneal epithelial staining—I’ll stain them with either rose bengal, lissamine green, or fluorescein stain—and the epithelium is showing signs of damage or injury due to their dryness, then I would avoid LASIK. If they’re not staining, but they’re just moderately dry, then I could consider something like punctal plugs, enhancing their artificial tears preoperatively or treating their eyelid disease. If there’s no epithelial staining I would do those measures and consider LASIK.”
 |
|
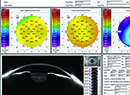
Irregular topography (A) due to dry eye should be treated prior to cataract surgery to help improve outcomes. After this patient’s ocular surface was treated, the topography was more regular (B) and contributed to more accurate calculations. Photo: Alice T. Epitropoulos, MD. |
Dr. Rubenstein tends to favor LASIK when possible on these patients. “An older patient may not heal well so PRK can be a harder operation for them to recover from,” he says. “Some people tend to do PRK in all of these patients, but I think LASIK works quite well. Some physicians worry that LASIK requires suction to hold the eye in place before creating a flap and there was concern that this could move the lens implant or harm the eye. With the femtosecond creation of a laser flap, I don’t think that’s an issue as far as disturbing lenses.”
Dr. Epitropoulos favors PRK. “I personally believe that a flapless procedure is generally less invasive, however, this perspective may not be universal among all practitioners,” she says. “I hold the view that PRK potentially reduces the risk of DED compared to LASIK, as LASIK involves cutting through the corneal nerves, which may contribute to dry-eye disease. While LASIK proponents argue for faster healing due to bypassing epithelial healing, I find that when the ocular surface is optimized most PRK patients heal quite rapidly, typically within three to four days.
“Following PRK, I employ a regimen involving a bandage contact lens, along with antibiotics (for a week) and steroids usually for a couple of months to minimize any haze or regression afterwards,” she continues. “When addressing refractive errors after cataract surgery, it’s common for patients to have minor residual refractive errors. In such cases, PRK is a suitable option. It’s worth noting that there are fewer concerns regarding corneal thickness limitations associated with LASIK in such cases.”
Dr. Berdahl was part of a study comparing post-refractive enhancements on IOL patients. A total of 822 post-cataract eyes underwent either LASIK or PRK. Sixty-seven percent of LASIK-enhanced patients achieved 20/20 or better post-enhancement UDVA, compared with 43 percent of PRK-enhanced patients. LASIK also had better outcomes when controlling for pre-enhancement UDVA, except in those with pre-enhancement vision of 20/20 or better, or those worse than 20/50.3
“We found that LASIK is more predictable than PRK and I think that’s likely because the epithelium is a bit more irregular by nature in patients that are older and hence, if you did a PRK instead of LASIK, you’d be removing that slightly irregular epithelium and treating the underlying stroma, but the underlying stromal refraction is different than the surface refraction,” explains Dr. Berdahl.
Considerations may also change if the patient had previous refractive surgery. These patients are more likely to need post-cataract enhancement, says Dr. Rubenstein. “Previous LASIK or PRK may take further LASIK enhancement out of the picture,” he says. “If you think their cornea is already thin or already very flat, you may not want to treat their cornea. If they’ve had LASIK that has any complications—whether it be flap complications, scarring, epithelial ingrowth or anything that could make their cornea less than perfect—I may not want to do further corneal refractive surgery on that patient. Therefore, that’s a patient on whom I may consider an IOL exchange if I don’t want to touch their cornea with refractive surgery.”
If the patient has undergone previous corneal refractive surgery, Dr. Epitropoulos says the age of a LASIK flap matters. “Having a flap makes it easy to lift it, perform ablation and then reposition the flap, resulting in slightly quicker healing for patients,” she says. “However, over time, the flap may become more challenging to lift, especially after several years. In these cases, performing surface ablation directly over the flap may be preferable.”
The surgeons we spoke with say they commonly target plano in these procedures, with some exceptions.
“I try to target plano unless the patient wants mild myopia for monovision or mini-monovision,” Dr. Rubenstein says. “You try to pick the refractive target after talking to the patient and finding out what their visual needs are. If the patient wants to be plano for good distance vision then that’s really what I aim for. If possible, try to leave them with a small degree of with-the-rule astigmatism versus against-the-rule astigmatism, because there’s a progressive drift towards against-the-rule astigmatism in patients as they age.”
“In general we target for plano OU for these patients with the exception if we’re trying to do a little mini-monovision with EDOF or LAL lenses,” Dr. Berdahl says. “The biggest technique for LASIK in these patients is just to be gentle. They’re more at risk for epithelial erosions. Then treat the ocular surface very aggressively in the postoperative period, especially if they’re having refractive surgery. Although I don’t personally use AKs frequently as a treatment in the postoperative period, I think that’s a reasonable treatment if your spherical equivalent is adequate and the benefit is that it doesn’t impact the epithelium.”
Other Ways to Correct
Surgeons say there are additional approaches available to you:
• Limbal-relaxing incisions. Dr. Rubenstein says this is an underutilized skill.
“It’s a skill I tend to be bullish about,” he says. “I’ve taught courses both at the American Academy of Ophthalmology and ASCRS meetings for many, many years on the skill of performing corneal-relaxing incisions. The best time to use this technique is if a patient has a postoperative spherical equivalent near plano, then you can go ahead and try to correct the astigmatism with peripheral corneal relaxing incisions.”
LRIs can be used to correct low errors, up to 2 D of astigmatism, he continues. “It’s usually rare to have postop patients who have more than that. Usually, you’re correcting 1 to 1.5 D of astigmatism with a spherical equivalent near plano. It’s a very effective technique. It can be done as an office-based procedure. Some people do this at the slit lamp.
“The technique and how much to cut is based on a nomogram, which requires an adequate refraction,” explains Dr. Rubenstein. “I’d refract the patient more than once at two separate visits to make sure I have a stable refraction. You can use a number of nomograms that are available in the literature and identify the amount of correction that needs to be performed. The level of treatment is based on the amount of astigmatism, the location of the astigmatism and the age of the patient. First, calculate how much astigmatism needs to be corrected and then it’s a minor surgical setup. I use the operating microscope with the patient supine, topical anesthetic, topical Betadine and then a diamond blade to create corneal incisions near the limbus at the peripheral cornea. You can either measure the peripheral corneal thickness with ultrasonic pachymetry and set your diamond knife to the thinnest of your measured thicknesses or use a preset 600 micron diamond blade.”
• IOL exchange. Thresholds for determining when it’s better to exchange the IOL differ.
For Dr. Rubenstein, an IOL exchange would be reserved for large residual refractive errors of 3, 4 or 5 D. “In general that’s unusual,” he says. “The misses are usually smaller and within 1 D. For these small refractive errors, I tend not to want to go back and exchange the lens.”
Dr. Berdahl says, “For those surgeons that don’t have access to an excimer laser, an IOL exchange or a piggyback or rotation of an IOL is a very reasonable option. There are many tools out there to help you figure out how to rotate the IOL to the proper location to minimize the amount of astigmatism. In my mind I philosophically moved away from thinking about an IOL exchange as a failure. It’s simply one more tool in our toolkit to get the patient to a happy spot.
“This is especially true as we’re pushing the limits of technology in eyes that are less pristine,” he continues. “This of course warrants careful informed consent with the patient’s understanding that their eyes aren’t pristine. But if they’re hoping for vision that’s free of spectacles or diminished use of spectacles and we think there’s a reasonable chance of achieving their visual goals, I offer this to the patient and we talk about the increased likelihood of an IOL exchange should they not be happy.”
Dr. Rubenstein prepares for a few scenarios as he proceeds with an exchange. “If you’re doing an IOL exchange, you’re not sure what’s going to happen when you get into surgery,” he advises. “You don’t know if that lens is going to come out smoothly or not. Your intent is to try to dissect the lens implant out of the capsular bag without any injury to the capsular bag or any injury to the lens zonules. That’s your number-one game plan. If you can do that, and you preserve the integrity of the capsular bag and zonules, you can put a replacement lens implant of the appropriate power back into the capsular bag. But that may not always be the case so you have to be prepared for scenario number two.”
Scenario number two is implanting a lens into the ciliary sulcus and capturing the optic with anterior capsular capture, he continues. “The optic stays in the capsular bag and the haptics are in the sulcus—that’s a very stable way of fixating the lens,” Dr. Rubenstein says. “The lens implant power ends up being basically the same as if the lens implant was fully in the bag.”
However, if the lens is out and the surgeon is worried about the bag and the zonules, they can try to put the lens implant into the sulcus alone and orient the lens implant in the position that gives the greatest amount of support with the existing zonules. “You usually have to change the lens implant power and decrease the lens implant power by 0.5 D to 1 D when you put the implant in the sulcus,” Dr. Rubenstein says.
“And then the fourth option, after you take the lens out and it turned out to be quite traumatic and you don’t have a good bag or sulcus, then you have to do some sort of fixation of the lens implant, either intrascleral haptic fixation or some sort of sutured fixation to the sclera,” he says. “When I take these patients in for lens implant exchange, I have all four options planned for. I have all the instrumentation for those four options lined up in the operating room and I’ll do whatever is necessary to produce a stable lens implant depending on what I’m encountering intraoperatively.”
• Repositioning the lens. Another scenario that can occur is if a lens implant is the correct power, but it’s out of position.
“For a toric lens, you may have to change the position of the lens implant so that it corrects the full amount of astigmatic power,” notes Dr. Rubenstein. “There’s a tool called Astigmatismfix.com, which allows you to assess the position that the lens sits in postop, and compares that with the intended position. The formula then calculates the anticipated reduction in the astigmatism if you rotate the lens and what position to rotate the lens to. You can also adjust the position of the IOL that’s currently in the eye in order to change the spherical power, such as bringing the optic outside of the capsule in a reverse optic capture. There are various manipulations of the existing lens implant that can sometimes give you a refractive change without having to exchange the lens entirely.”
• Piggyback. If the refractive error produced hyperopia in the patient, a piggyback lens may be considered.
“A piggyback lens implant can go on top of the lens if the intraocular situation is deemed to be safe: an adequate anterior chamber; a healthy cornea and corneal endothelium; and a well-placed initial lens implant that allows you to piggyback the new lens implant in the ciliary sulcus on top of the existing lens implant,” says Dr. Rubenstein.
Patient Relationships
Dr. Epitropoulos says if these measures don’t resolve a patient’s problem, then they should be thoroughly evaluated, including topography, followed by an OCT to rule out macular edema.
Often, these challenging patients can be identified and counseled ahead of surgery. “During discussions with patients, I emphasize that while we employ advanced equipment and techniques, the human body’s response to surgery can be unpredictable despite our best efforts,” she says. “This perspective helps to provide context for surgical outcomes and underscores the fact that results aren’t solely determined by the surgeon’s skill. I also highlight the remarkable healing and adaptive capabilities of the human body, reassuring patients that their bodies have the capacity to respond positively to treatment with time.
“I train my staff to be vigilant in identifying patients who may have unrealistic expectations or whose needs we may not be able to fully meet,” continues Dr. Epitropoulos. “This proactive approach helps ensure that we can manage patient expectations effectively and provide the best possible care for each individual. By identifying such patients early on, we can have open and honest discussions with them about what they can realistically expect from their treatment, thereby minimizing the likelihood of dissatisfaction or misunderstanding later.”
Dr. Berdahl has relationships with Alcon, Zeiss, Johnson & Johnson Vision, Bausch + Lomb, RxSight, Astigmatismfix.com and ExpertOpinion.MD. Dr. Epitropoulos consults for Alcon, Bausch + Lomb, SightSciences and is a consultant/chief medical officer for PRN Vision. Dr. Rubenstein is a consultant for Alcon.
1. Khoramnia R, Auffarth G, Łabuz G, Pettit G, Suryakumar R. Refractive outcomes after cataract surgery. Diagnostics (Basel) 2022;19:12:2:243.
2. Berdahl JP, Hardten DR, Kramer BA, Potvin R. Effect of astigmatism on visual acuity after multifocal versus monofocal intraocular lens implantation. J Cataract Refract Surg 2018;44:10:1192-1197.
3. Rohlf D, La Nasa A, Terveen D, Shafer B, Thompson V, Berdahl J. Outcomes of LASIK vs PRK enhancement in eyes with prior cataract surgery. J Cataract Refract Surg 2023;1:49:1:62-68.