As the ophthalmic R&D pipeline pushes out more minimally invasive glaucoma surgery devices, ophthalmologists are combining them in innovative and sometimes aggressive ways to lower IOP, reduce medication burden and help patients tolerate their disease. However, guidance on how to blend these diverse technologies is in short supply.
Because competing manufacturers don’t team up to fund randomized clinical trials that support universal guidelines, many surgeons are left to conduct their own retrospective studies to inform their use of MIGS combinations. Most of the choices on when and how to combine MIGS are also driven by first-hand insights on what seems to work best.
Here, several glaucoma specialists explain why they combine MIGS procedures and which specific procedures they use to achieve their objectives.
Combining MIGS: Why and When
Although MIGS are combined in standalone procedures, the classic definition of combination MIGS includes the use of phacoemulsification and two or more MIGS procedures, according to David Solá-Del Valle, MD, a glaucoma and cataract specialist, chief medical officer at Chittick Eye Care, and associate Professor at the Carle Illinois College of Medicine in Champaign, Illinois.
Dr. Solá-Del Valle recently led a comprehensive review of studies on MIGS combinations.1 When selecting a patient, he first determines if one MIGS modality alone is appropriate.
“If we can agree on that, then we can explore who would be good candidates for combined MIGS,” explains Dr. Solá-Del Valle, who also conducts part-time research at Massachusetts Eye and Ear as a former assistant professor at Harvard Medical School.
And when does he combine MIGS? “Let’s say the patient’s goal pressure is 17 mmHg, and the pressure is above 20 mmHg—at 23 mmHg or 24 mmHg, for instance,” he explains. “The patient is on two or three medications and wants to end or significantly decrease his medication burden. I find that single MIGS would have difficulty achieving this goal, which would enable the patient to stop using all agents, for example. So combination MIGS will be a great option for that patient, who may have mild-to-moderate disease.”
 |
|
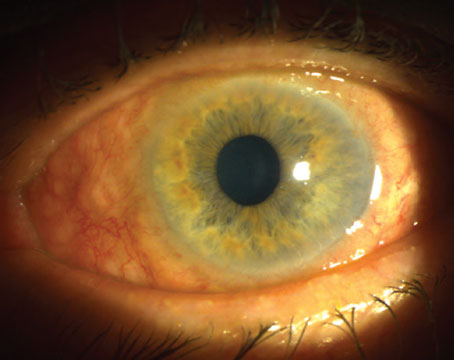
Combining ab interno canaloplasty (shown here) with stents is a favored combination by some surgeons, including those considering this approach as a standalone combination in uncontrolled patients who don’t require cataract surgery. Photo: Matt Porter, OD. |
Last Ditch Effort?
Although MIGS combinations are usually used early in disease, Derek Mai, MD, a glaucoma specialist at Madigan Army Medical Center in Tacoma, Washington, sometimes uses them in advanced glaucoma cases. Many patients in his military-based practice have had surgeries 20 to 30 years before he even sees them.
“Some are still progressing,” he says. “I will have a pretty in-depth discussion with these patients about what they can expect, including limitations. If they have an open angle, and they don’t have any contraindications for an endocyclophotocoagulation procedure, I discuss combining different MIGS. Combined MIGS in these patients entails long-term inflammatory risks and an immediate risk of hyphema developing right after surgery. But I’ve had some very good successes, in terms of improving a patient’s quality of life, reducing medication burden and lowering IOP into the low to mid-teens.
 |
|
Combining phaco, endocyclophotocoagulation, and goniotomy using the Kahook Dual Blade—a combination known as PEcK—enables the surgeon to increase trabecular outflow while decreasing aqueous production. Photo: David Solá-Del Valle, MD. |
“The literature may indicate there is minimal evidence to support combined procedures for these advanced patients,” he continues. “But when there are no other options left, I find myself using these combinations as a last-ditch effort to get their glaucoma under control.”
Dr. Solá-Del Valle also uses combined MIGS in patients with moderate-to-severe disease, defined by the ICD-10 codes. “I’m thinking of a patient with small nasal steps such as a superonasal step with an early inferonasal step that may not always be repeatable,’’ he notes. “Combined MIGS can be a stepping stone before moving on to more invasive procedures.”
After combined-MIGS, some patients can still experience glaucoma progression and a need for multiple medications. But without the option of combined MIGS, Dr. Solá-Del Valle adds, “that patient may have otherwise undergone a trabeculectomy or a tube shunt earlier on. Nonetheless, I still avoid combined MIGS in extremely advanced disease, such as patients with central islands of vision or those who need single-digit IOPs.”
 |
|
Some surgeons have used phaco with two trabecular meshwork procedures, the iStent Inject and the iAccess, a combination known as Pi3. Photo: David Solá-Del Valle, MD. |
Only the Beginning?
Matt Porter, MD, a cataract and glaucoma surgeon and an assistant professor at Texas Tech University Health Sciences Center School of Medicine, is also bullish on combining MIGS, an approach that he believes will become more prevalent in the future. “This is a subject that is somewhat under-explored in the literature,” he says. “It has a lot of potential to help patients, and it’s important to talk about it and get people thinking about it.”
Dr. Porter has tried varied combinations of MIGS but he’s most comfortable using the Hydrus (Alcon)—an eyelash-sized stent for mild and moderate glaucoma—in conjunction with the iTrack Advance (Nova Eye Medical), which provides 360 degrees of catheterization and pressurized viscodilation in the conventional outflow pathway.
“It’s not the only combination that I use,” he explains. “But it’s the most common one. My colleagues, Mark Gallardo, MD, Brandon Wood, MD, and I collected our own data on this, and we’ve been very pleased with strong results several years out. When you see your patients are still doing well, it’s hard to argue with the results, especially in the absence of randomized controlled trials.”
Their retrospective evaluation of first-year results was based on 51 patients, 41 of whom were uncontrolled—defined as any patient with an average baseline IOP of 19 mmHg who was taking a mean of 2.2 medications.
“A lot of these patients were very sick,” he notes. “That’s significant when you consider that most patients in typical MIGS trials have mild to moderate glaucoma. Three-quarters of our patients were actually either moderate or severe. At one year, their IOP was down to an average of 13 mmHg, while they were taking only one medication. That’s a significant reduction in pressure and medication burden.”
Findings in the study’s uncontrolled patients, whom he would have otherwise treated with a tube shunt or a trabeculectomy, have also continued to be strong. “In fact, the uncontrolled patients did just as well or better than more controlled patients in the study,” he points out. “They had a higher starting IOP of 19.9 mm Hg and were using an average of 2.4 medications with similar results as was experienced by the total group. If we can combine different mechanisms of action, we can potentially improve the results for these patients.”
He notes that Hydrus scaffolds the canal, providing multiple openings over a quarter of the canal’s circumference. “When combining Hydrus with canaloplasty, the result is use of two procedures that are complementary, attacking both the proximal and distal conventional outflow systems,” he says. “Bypassing the trabecular meshwork and treating the distal canal and collector channels might increase chances of success without having to excise the trabecular meshwork. Adding stents to canaloplasty can perhaps make it work longer and more effectively. In my experience, a moderate open-angle patient with an IOP in the mid 20s, on three or four medications, could benefit from these combination procedures.”
Dr. Porter also hopes to try a stand-alone combination by using stents and ab interno canaloplasty in uncontrolled patients who don’t require cataract surgery. He plans on combining ab interno canaloplasty with iStent Infinite (Glaukos) stents as his choice of a standalone combination approach.
 |
|
Presented are the diagnostic findings of a 77-year-old female with primary open-angle glaucoma who was experiencing difficulty driving at night. She underwent a combination MIGS procedure known as ICE2, which includes iStent inject, cataract surgery and endocyclophotocoagulation. After the procedure, her BCVA improved from 20/150 to 20/30. Her anti-glaucoma medications decreased from two to one, and her IOP decreased from 17 mmHg to 11 mmHg. (Two images were combined to show the pertinent findings.) Photo: David Solá-Del Valle, MD. |
Combining the Right MIGS
Many surgeons develop favored lists of MIGS procedures to combine, including the use of Xen (Allergan), a subconjunctival-based intervention. Like Dr. Porter, Dr. Solá-Del Valle mixes his preferred options to take advantage of different mechanisms of action for moderate-to-severe patients. “I’m a big fan of doing phaco with ECP and goniotomy, using the Kahook Dual Blade, the SION device, or gonioscopy-assisted transluminal trabeculotomy (GATT), Omni, or a similar procedure,” he says. “I find that these are amazing combinations because you’re opening the drainage system via the trabecular meshwork with the goniotomy, for example. But you’re also using the ECP laser to decrease fluid production.”
 |
As an alternative to ECP, Dr. Solá-Del Valle says micropulse transscleral cyclophotocoagulation can be helpful. He notes that he participated in a study2 at his institution that compared the micropulse laser combined with phaco to ECP combined with phaco.
“We found that the transscleral group did just as well as the group in which phaco and ECP were used—if not just as well, even a little better than the ECP group, depending on which factors we considered,” recalls Dr. Solá-Del Valle. “Keep in mind that you can combine either of these procedures with any number of trabecular meshwork procedures (such as iStent, or the ICE procedure, which combines iStent, phaco and ECP), as well as with iAccess, Hydrus, and other stents. The possible MIGS combinations can seem endless, especially with all the new MIGS in the pipeline.”
He points out that two trabecular meshwork-based procedures can also be combined, especially for patients with less severe glaucoma. “I’m thinking of Streamline canaloplasty with iStent Infinite or iStent Infinite with iAccess,” he explains. “Success depends on individualizing patient care by considering patient characteristics, goals of care and surgeon experience.”
Dr. Solá-Del Valle and his colleagues presented the findings of an ongoing study at the 2024 American Society of Cataract and Refractive Surgeons in Boston that showed how adding iAccess to the ICE-2 procedure (iStent Inject, cataract extraction and ECP) seemed to reduce the medication burden at six months.
Reality Check
Like all combinations of glaucoma treatments and surgeries, combinations of MIGS are far from perfect.
“There’s currently a lack of evidence or formal studies regarding the combination of MIGS procedures,” says Reza Razeghinejad, MD, associate professor of ophthalmology and director of the glaucoma fellowship program at Wills Eye Hospital in Philadelphia.
Dr. Razeghinejad, who combines MIGS for some of his patients, says these procedures can sometimes create uncertain effects when they bypass the trabecular meshwork. “Our approach (when using MIGS) assumes that the drainage system distal to Schlemm’s canal is functioning properly,” he observes. “However, in most glaucoma patients, the primary resistance appears to be beyond Schlemm’s canal rather than at the trabecular meshwork. If we perform MIGS in a quadrant with functioning aqueous veins, then we can reasonably expect a fair reduction in IOP.” The challenge, however, is achieving this type of success predictably, Dr. Razeghinejad adds. “Unfortunately, the gold standard for understanding drainage system function—aqueous angiography—is not yet commercially available,” he says.
Reviewing today’s available combined MIGS procedures, Dr. Razeghinejad notes that Schlemm’s canal dilation and procedures that bypass the trabecular meshwork are very common. Schlemm’s dilation, achieved by the Omni Surgical System (Sight Sciences) and Streamline (New World Medical), is designed to create precise goniotomies in the trabecular meshwork and deliver small amounts of ophthalmic viscosurgical device (OVD) into Schlemm’s canal, explains. Other options he mentions: Ab interno canaloplasty and the bypassing of the trabecular meshwork by stenting or performing a goniotomy.
Combining MIGS with ECP or micropulse cyclophotocoagulation actually raises a concern, he notes. The combinations can “potentially increase postop inflammation and lead to cystoid macular edema,” he adds.
“Suprachoroidal implants are considered MIGS procedures, but the primary concern lies in corneal endothelial loss,” he says. “We eagerly await the results of trials on Miniject (iStar Medical) and a possible bio-tissue stent in the future to assess the impact on endothelial cells. Combining suprachoroidal and angle-based MIGs appears promising if suprachoroidal implants become available.”
 |
Complications to Consider
One of the more common complications of combing MIGS is hyphema, which is typically microscopic but can occasionally be macroscopic and associated with elevated IOP, according to Dr. Razeghinejad. “While most patients will experience reabsorption of the hyphema, there are cases where anterior chamber washout, with or without filtering surgery, may be necessary,” explains. “To reduce the risk of hyphema, I remove viscoelastic material several minutes after completing the MIGS procedure, allowing it to have a tamponading effect on the refluxing blood. Additionally, I maintain the IOP within the range of 20-25 mmHg at the end of surgery to further decrease the chance of hyphema. It’s important to note that patients undergoing MIGS typically have an optic nerve capable of handling this IOP.”
Cyclodialysis clefts are increasingly observed as a complication following MIGS, says Dr. Razeghinejad. “The attachment of the iris to the sclera is not firm,” he notes. “Any trauma to the iris could lead to cleft formation. To decrease the chance of this complication, the anterior chamber may be filled with cohesive OVD (viscoelastic) during surgery. This helps maintain the depth of the anterior chamber and avoid forward movement of the iris,” he says.
Looking Ahead
Complications aside, many surgeons remain optimistic about combining MIGS. “The beauty of glaucoma in 2024 is that there are so many groups all over the world trying to come up with better treatments for our patients,” says Dr. Solá-Del Valle. “It’s now our job to match the best treatments to the best patient. That’ll be the challenge for all of us in the future. Hopefully, more research will help guide us in this endeavor.”
Dr. Mai has no financial relationships with ophthalmic companies. Dr. Porter is a consultant with Glaukos. Dr. Solá-Del Valle has financial relationships with Allergan (an AbbVie company), having received fees for lecturing on the XEN Gel Stent in 2021 and continuing to serve as a consultant in 2024. He also received lecture fees from New World Medical in 2024, and he served as a consultant for BVI Medical, Lensar (Ally Adaptive Cataract Treatment System), and Théa Pharma, Inc. in 2023. Dr. Razeghinejad has been involved in research grants from Equinox and Olleyes that were awarded to the hospital where he works. The Equinox relationship has ended.
U.S. Military Disclaimer
The identification of specific products and scientific instrumentation in the preceding article is considered an integral part of scientific endeavor and does not constitute an endorsement or implied endorsement on the part of Derek Mai, MD, on the part of the Department of Defense, or any other public agency. The views expressed in Dr. Mai’s statements are totally those of Dr. Mai and do not reflect the official policy of the United States Army Medical Command or Department of Defense.
1. Mai DD, Ingram Z, Oberfeld B, Solá-Del Valle D. Combined microinvasive glaucoma surgery – A review of the literature and future directions. Seminars in Ophthalmol 2023; 38:6:529-536.
2. Nirappel A, Klug E, Neeson C, et al. Transscleral vs endoscopic cyclophotocoagulation: Safety and efficacy when combined with phacoemulsification. BMC Ophthalmol 2023;23:1:129.
3. Ianchulev TA, Weinreb RN, Kathan G, et al. Biotissue stent for supraciliary outflow in open-angle glaucoma patients: Surgical procedure and first clinical results of an aqueous drainage biostent. Br J Ophthalmol 2-24;108:2:217-222.