Minimally-invasive glaucoma surgery is intended to lower intraocular pressure with less tissue disruption than traditional glaucoma surgeries. Current options include canal-based stenting (iStent by Glaukos and Hydrus by Ivantis), viscodilation devices (Omni by Sight Sciences and ABiC by Ellex), excisional goniotomy (Kahook Dual Blade by New World Medical), and a cautery device (Trabectome by MicroSurgical Technology). (The Allergan Xen was voluntarily recalled in October 2019, and surgeons were told not to implant more of them; there’s been no word when surgeons can purchase new ones to implant.)
With a variety of options—and mechanisms of action—to choose from ophthalmologists can sometimes be hard-pressed to make sense of it all. Here, glaucoma experts share their thoughts on how they use the available devices, and we take a look at some of the results the devices are capable of achieving.
Making Sense of MIGS
“The angle-surgery space is extremely crowded now. It used to be a discussion about iStent vs Trabectome,” says Malik Kahook, MD, a professor of ophthalmology at the University of Colorado School of Medicine. “Now, there are a dozen different approaches and a growing body of evidence for most of the devices we use today. It’s clear to me that each company is trying to position its device based on safety and efficacy; however, the economics of the space (device cost and reimbursement) is also playing a major role that may be underrepresented in many of our discussions at meetings and in trade journals.”
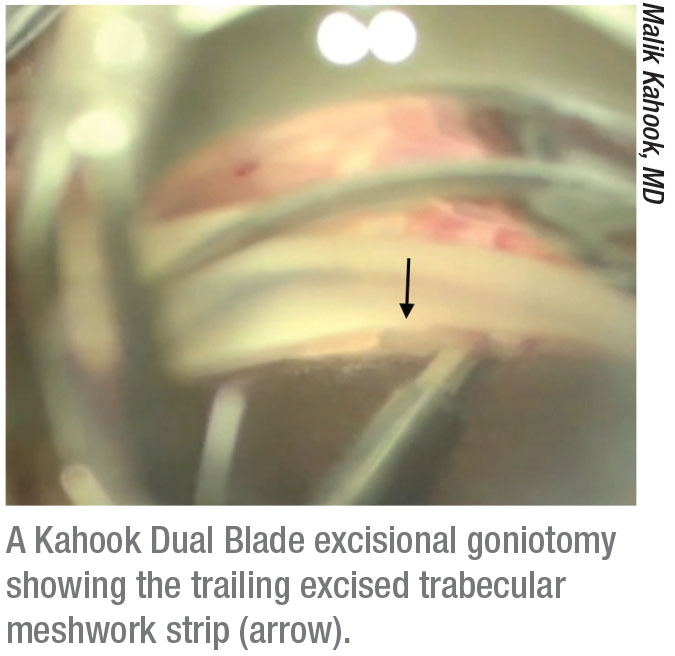 |
According to E. Randy Craven, MD, associate professor and director of the Wilmer Eye Institute, Johns Hopkins Medicine satellite office in Bethesda, Maryland, when evaluating glaucoma patients, ophthalmologists first need to consider whether or not they’ll be undergoing cataract surgery. “For people who aren’t undergoing cataract surgery, but who have elevated IOP and for whom medications aren’t working, I look at their age, refractive status, angle anatomy and ocular history,” he says. “For example, I note if someone has a history of long-term steroid use or uveitis. Then, I review the options and try to make a plan for the patient.
“Some surgeons use trabecular bypass procedures off-label for these patients,” he continues. “I had a chance to do that in Saudi Arabia where it’s approved. In the future, that might be an option for us, but in the meantime, we’re left with having to remove the trabecular meshwork either through a goniotomy with some kind of a blade procedure or ab interno trabeculectomy with a catheter. That approach is kind of between a goniotomy/trabeculotomy, with the idea being that you remove the trabecular meshwork, expose the canal, and try to get the pressure down,” he explains.
He says he leans towards these hybrid procedures in high myopes to avoid filtering procedures and tubes. “I’ve had good success with all of the blade procedures and goniotomy procedures in high myopes, especially if they have a moderately high IOP. Patients who have a history of chronic steroid usage or uveitis are also good candidates for this procedure,” he says.
For patients who undergo cataract surgery, Dr. Craven says he still can’t predict whether or not a trabecular bypass procedure is going to work. “I like the bypass procedure better than an extirpation procedure and combination procedures because, to me, the meshwork might hold some mechanism of action in IOP reduction,” he says. “I think the meshwork actually plays a role in helping to force aqueous out to the collector system, so I don’t know if I want to remove it. I like the Hydrus, and I’ve implanted it on many continents. I’ve probably done 100 of them post-approval here. It’s a fairly straightforward procedure to teach my fellows.
“The other big advancement in that same area has been the iStent inject because it’s a lot easier for people to put in and, with the two stents in, I think we’re seeing better pressure reduction,” Dr. Craven adds. “I like the ability of those stents to be implanted in patients who might have a more crowded angle,” he continues.
Outcomes
According to Dr. Kahook, a simple literature review shows that most angle devices, whether stent implants or goniotomy devices, produce essentially the same IOP lowering after long-term follow-up. “Some devices might have enhanced efficacy in particular situations—for example, goniotomy with angle-closure glaucoma or post failed iStent implantation—but they all work almost equally well in mild to moderate primary open-angle glaucoma when combined with cataract extraction,” he says. “I use the Dual Blade for my angle procedures, and sometimes combine it with endocyclophotocoagulation, which I consider a minimally invasive approach. I choose it because I’ve had the most experience with it and it works best in my hands,” he explains.
In terms of the latest iStent iteration, there’s recent data from Europe on the iStent inject.1 In a prospective, non-randomized, consecutive case series, researchers operated on eyes with primary open-angle glaucoma, in addition to other types. All eyes underwent ab interno iStent inject implantation as a sole procedure. Assessments over three years included IOP measurements, medications, corrected distance visual acuity and secondary glaucoma surgeries. Two iStent inject stents were implanted in 44 consecutive eyes (POAG=38, PXG=4, appositional NAG=1, secondary neovascular glaucoma=1) of 31 patients, and 33 eyes had 36-month follow-up data. Preoperative mean IOP was 25.3 ±6.0 mmHg on a mean of 2.98 ±0.88 medications, with three-quarters of the eyes on three to five medications (no eyes were medication-free). Half of the eyes had a history of prior glaucoma surgery. (Glaukos funded the article processing and provided writing assistance for the study.)
At three years postop, the mean IOP was reduced by 42 percent to 14.6 ±2.0 mmHg (p<0.0001) and 87.9 percent of eyes achieved an IOP reduction of ≥ 20 percent. In addition, 97 percent of eyes reached an IOP of ≤ 18 mmHg (vs. 9.1 percent preoperatively; p<0.0001) and 70 percent of eyes reached IOP ≤ 15 mmHg (vs. 2.3 percent preoperatively; p<0.0001). Mean medication burden decreased by 82 percent to 0.55 ±0.79 (p<0.0001), and 61 percent of eyes were medication-free. All eyes maintained or decreased their 36-month medication burden versus preoperative levels. The researchers say there were “minimal” adverse events and CDVA was stable through 36 months.
A different study looked at the Kahook Dual Blade. The retrospective study from Texas included 111 eyes of 90 patients who underwent KDB goniotomy from January to November 2016. KDB goniotomy was combined with cataract surgery in 100 eyes.2 The main outcome measures were postop IOP and the number of IOP lowering medications.
Preop, the mean IOP was 17.1 ±4.7 mmHg and the mean number of glaucoma medications was 2.4 ±1.3. Postop, mean IOP was 14.9 mmHg, 13.9 mmHg, 14.1 mmHg, 14.4 mmHg, and 14.7 mmHg at one, three, six, nine and 12 months follow-up, respectively (all p<0.004). The mean number of IOP-lowering drugs was 0.8, 1.0, 1.0, 1.0, and 1.6 at one, three, six, nine and 12 months follow-up, respectively (all p<0.001).
The cumulative reoperation rates for uncontrolled IOP after KDB were 0, 1, 2.1 and 4.6 percent at three, six, nine and 12 months, respectively. Eyes with a preoperative IOP >21 mmHg were significantly more likely to undergo reoperation (p=0.038). There were no serious complications at any time point in the follow-up period.
“Often, when surgeons are adept at many of these techniques, it comes down to economics,” says Dr. Kahook. “The physician reimbursement for 0191T (iStent and Hydrus) has been decreased by Medicare Administrative Contractors across the country. Category I codes, used for goniotomy (65820) or viscocanalostomy (66174), have more consistent reimbursement numbers. It should also be noted that the ambulatory surgery center facility fee for some Category I code procedures is not as high as the device intensive codes that are currently coupled with 0191T devices. However, hospital-based facility fees are relatively uniform across most angle procedures regardless of type. Each center has to figure out the economic viability of each approach while, of course, always choosing what they believe is the best approach for each patient given individual surgeon experience and outcomes.”
He adds that the value proposition for all angle-based procedures is enhanced safety compared to full-thickness procedures. “I choose angle procedures when I want low to mid-teen IOP and for patients in whom the major goal is decreasing their dependence on medications. If I need sub-12 mmHg pressure, I still rely on trabeculectomy, ExPress (Alcon), and drainage devices such as the Ahmed Glaucoma Valve and the Ahmed ClearPath (New World Medical),” Dr. Kahook says.
“I think the innovation represented by these new devices and procedures has sort of outstripped our ability to fully assess the effects of each one,” says Leonard K. Seibold, MD, an associate professor of ophthalmology at the University of Colorado School of Medicine. “As we use each procedure, we’re learning more about it. But for me personally, I use goniotomy or the Kahook Dual Blade in a lot of scenarios because it’s probably the most versatile procedure. You can use it in different disease severities. It doesn’t require leaving an implant in the eye, and it can be used for different types of glaucoma.”
The prospective HORIZON trial, sponsored by Ivantis, analyzed the Hydrus device when used in conjunction with cataract surgery, with cataract surgery alone used as a control. The study included 556 patients and spanned 38 centers in nine countries. The three-year data from the study found:
• 73 percent of Hydrus Microstent patients remained medication-free, compared to 48 percent in the cataract-only arm;
• Among patients who entered the study on one medication, 81 percent were medication-free, compared to 48 percent in the cataract-only arm;
• Only 0.6 percent of Hydrus patients went on to have invasive glaucoma surgery, compared to 3.9 percent in the cataract-only arm, an 85-percent reduction.
• An overall safety profile that was similar to cataract surgery alone.3
Atlanta’s Reay Brown, MD, says he’ll use devices or other approaches when appropriate. “The data from the device studies show 1.6 and 2.2 as the delta vs. cataract surgery,” he says. “That’s a lot of work and explanation to patients for a relatively small change in pressure. But, I’m a MIGS optimist, so I still will use a device or do an Omni in essentially every case in which I’m doing cataract surgery on a patient who’s on glaucoma drugs. Their pressures come down, and everybody’s happy, but we don’t know how much of the pressure lowering is from the minimally-invasive glaucoma surgery and how much is from removing the cataract. In my practice, I’m doing more trabeculotomy/canaloplasty, because I seem to get more pressure lowering than with the devices,” he explains.
The Future
Dr. Seibold believes that MIGS procedures will gain popularity. “Glaukos and others are developing suprachoroidal stents, so I think we’ll see the suprachoroidal pathway come back as another option,” he says. “As the burden of glaucoma increases and drug costs continue to climb, MIGS is a great option for these patients to help better control their disease with fewer or no medications, and I think eventually that’s going to be seen as a cost savings for patients. It’s certainly a quality-of-life boost. Also, residency programs are increasingly training residents in MIGS, so new ophthalmologists are coming out of training more comfortable with doing an angle-based or MIGS procedure,” he adds.
Dr. Brown emphasizes that now the various approved options are being tested. “We’re trying different things and seeing what works best,” he notes. “It’s very empirical. We’re trying to find out how to use what we have—and we have the most options that we’ve ever had, by far. So, it’s a little bewildering, but each surgeon is doing his or her own empirical testing. This is our best option, because there are no definitive studies comparing them to each other.” REVIEW
Dr. Kahook receives royalties from and consults for Alcon, New World Medical and J&J Vision. He has equity in Ivantis. Dr. Brown is chief medical officer of Sight Sciences. Dr. Craven is a consultant to Allergan, Alcon, Ivantis and Transcend. Dr. Seibold is a consultant for New World Medical and Allergan, and has received research support from Alcon and Glaukos.
1. Hengerer FH, Auffarth GU, Riffel C, et al. Second-generation trabecular micro-bypass stents as standalone treatment for glaucoma: A 36-month prospective study. Adv Ther 2019;36:7:1606-1617.
2. Kornmann H, Fellman R, Feuer W. Early results of goniotomy with the Kahook Dual Blade. Clin Ophthalmol 2019;13:2369.
3. 3-year HORIZON results. Data on-file, Ivantis.



