Mark B. Abelson, MD, CM FRCSC, Daniel Dewey-Mattia, and Aron Shapiro, North Andover, Mass.
Acanthamoeba keratitis is a rare sight-threatening disease which, in the United States and Europe, is found predominantly among contact lens wearers.1,2 The condition is caused by infection of the protozoa Acanthamoeba and is commonly misidentified as one of the more common fungal or viral forms of keratitis, especially ocular herpes.3 Accidental corneal trauma, which is more common in developing countries, and contact lens wear are the primary risk factors for AK. Treatment methods are most successful during early stages, so prompt diagnosis is crucial to halting disease progression. Later-stage patients are often faced with a corneal transplant as their only means of regaining a visual acuity of more than simply hand movements;4 thus, AK should be a significant concern among contact lens wearers and individuals who have recently experienced surgical or accidental corneal abrasions. Prevention is the best weapon against AK, as most cases can be directly attributed to poor contact lens hygiene or direct eye contact with contaminated water.
Acanthamoeba Ecology
Acanthamoeba is one of the more abundant protozoa on earth. Members of the genus Acanthamoeba are almost ubiquitous and have been isolated from soil, dust, air, treated and untreated tap water, swimming pools, air-conditioning units and numerous other domestic and outdoor environments.5 The protozoas' life cycle consists of an active feeding trophozoite phase and dormant cyst phase, which is activated by unfavorable conditions, such as exposure to extreme temperature, high or low pH, dryness or starvation.5 Resilience through adverse conditions (cysts can remain viable for 24 years or longer)6 allows Acanthamoeba a broad ecological range and also makes it a formidable parasite in humans.
Since Acanthamoeba is so prevalent in our environment, it's surprising that infections are so uncommon. It is not known how our immune systems protect us from infection, although Acanthamoeba-specific antibodies have been discovered in humans.3 Evidence of Acanthamoeba has been found in lung and nasal tissue of both sick and healthy individuals7 and as an opportunistic pathogen Acanthamoeba has been known to cause fatal encephalitis in immunocompromised patients.5 In AK, however, infection typically occurs in immunocompetent individuals, although some research suggests that some AK patients may be susceptible to the pathogen due to a lack of antigen-specific antibodies.3
Amoebic Keratitis
An estimated 5,000 cases of Acanthamoeba keratitis have occurred in the United States as of 2006, but since AK is so commonly misdiagnosed and the disease doesn't need to be reported to the Centers for Disease Control and Prevention, the actual number could be much higher.3 Developing nations in Asia and Africa, where AK is usually not associated with lens wear, have an exceptionally high rate of infectious keratitis. One study of non-contact lens wearing patients in India reported a very poor final visual outcome compared to previous research among lens wearers in Western countries.8 This may be due to variation among geographically distinct Acanthamoeba strains or merely the lower socioeconomic status and lack of medical resources available to study participants.8
AK was first documented in the
Beginning in the mid-1990s the 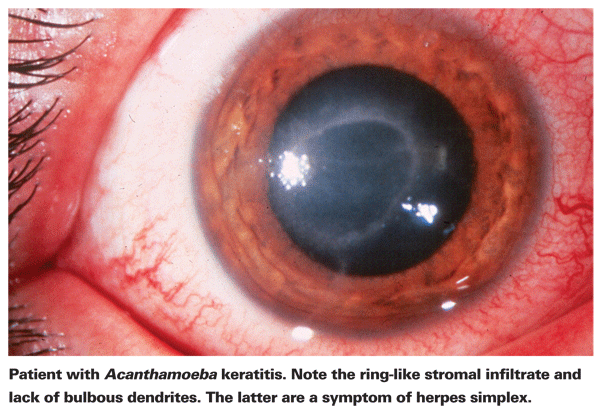
Studies have begun to show that community water storage and treatment may also play a large role in AK outbreaks. In England, where AK incidence is 15 times that of the United States, infrequently flushed-out roof-top water tanks may serve as excellent media for Acanthamoeba growth and may explain the country's bloated AK rates.17 Investigation after the Chicago area outbreak found that not only did the increase in AK correspond to a lowering of city water chlorination following an Environmental Protection Agency mandate regarding carcinogenic byproducts, but the exceptionally long distances of municipal water travel in the area may have allowed time for additional dechlorination and Acanthamoeba colonization.12 Even though many water treatment procedures aren't effective at destroying cysts, disinfectants such as chlorine do effectively destroy the free trophozoites and inhibit protozoan growth and reproduction.12
Contact Wear and Corneal Tear
Corneal trauma, ocular surgery and contact lens use are all risk factors for the development of infectious keratitis.17 When the epithelial layer of the cornea is damaged, it is left susceptible to infection by fungi, bacteria or protozoa.18 Prior to the widespread use of contact lenses, corneal abrasions and exposure to contaminated water or soil were the most distinguishable risk factors for AK.
Contact lenses can help Acanthamoeba reach the cornea and exacerbate the progression of the disease. The lenses serve as a physical route of passage from a contaminated source (i.e., lens storage case or tap water) directly to the cornea. Lenses, especially when worn improperly or for longer than recommended, erode the corneal epithelium, allowing microbial entry.19
Lens wear is also associated with upregulation of mannosylated receptor proteins on the epithelial surface that augment Acanthamoeba's ability to eat away the corneal tissue.19 Biofilms and deposits of mannosylated proteins that build up on the lens surface cause trophozoites to produce increased amounts of epithelial apoptosis mediators and proteases that degrade the corneal stroma's matrix.19
Susceptibility to Acanthamoeba infection also depends on what type of contact lens an individual uses. Overnight lenses put users at a drastically greater risk of developing AK, as one review found a 30-percent prevalence of the disease among users.20 While daily disposable lenses are the type of lens least likely to lead to infection,21 individuals who wear rigid lenses are less prone to AK than users of soft lenses.22 Approximately 88 percent of contact lens-related AK cases occur in soft contact lens wearers and 12 percent in rigid lens wearers.23 Increasingly popular continuous-wear silicon hydrogel lenses are also an easier target for Acanthamoeba attachment than conventional hydrogels.21
Clinical Signs and Symptoms
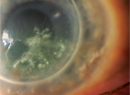
Sufferers of AK typically complain of redness, pain, decreased visual acuity and sensitivity to light.5 The most distinctive clinical feature of AK is a pronounced ring-like stromal infiltrate, believed to be composed of inflammatory cells.5 Signs of scleritis, corneal inflammation and conjunctival hyperemia begin to appear as the disease progresses.5 If left untreated, Acanthamoeba can spread back into the retina and cause serious chorioretinitis,5 and patients are often reduced to a visual acuity of hand movement or blindness in these later stages. Late diagnosis, a pronounced ring infiltrate and deep stromal infection are all associated with a poorer final visual outcome.24 Patients who develop stromal keratitis are also more likely to have a poor visual outcome after treatment than those with a more superficial epithelial infection.4
Diagnosis
Proper early stage diagnosis is essential before the Acanthamoeba invades more deeply into the cornea and causes permanent tissue damage. Unfortunately, at the initial visit the disease is more commonly diagnosed as herpes simplex keratitis than AK. This misidentification can lead to the use of antiviral medication, which only makes symptoms worse for the patient. During diagnosis, Acanthamoeba is often considered as a cause of infection when corneal ulcers are unresponsive to antibiotics. This delay in proper diagnosis allows the parasite ample time to progress further into the eye.
The first step towards proper diagnosis is accurate slit-lamp examination. The slit lamp can show evidence of Acanthamoeba either through its direct presence or by the associated immune response.24 The lack of bulbous dendrites typical in herpes patients is a good indicator a patient might have AK. Early and accurate slit-lamp examination is important in corneal ulcer patients, since disease stage at the time of AK diagnosis is a strong predictor of final visual outcome.24
When available, direct in-vivo diagnosis can be performed using an advanced tandem scanning confocal microscope and Heidelberg retina tomograph II (HRT-II) with cornea module.7 Polymerase chain reaction can also be used as a diagnostic tool when too few cells are available for an accurate visual determination.5 If in-vivo examination and PCR aren't feasible, culturing corneal scrapings can be an invaluable tool in diagnosis of Acanthamoeba and differentiating it from ocular herpes.
Corneal scraping with a potassium hydroxide wet-mount preparation should be used if microscopic examination is possible.25 Additional scrapings should be performed using sterile equipment until there is sufficient material for culture plates. Samples should be incubated at approximately 37 C on a non-nutrient agar plate coated with Escherichia coli.24 Within days, a positive culture will be covered with Acanthamoeba feeding on the bacterial overlay.3
Treatment
Acanthamoeba is difficult to treat, but effective management can keep a patient from going blind in (at least) one eye. Topical anti-infectives typically only work in early-stage patients, while surgical intervention is necessary in later stages. In 1985, the first successful treatment of AK was reported with the use of propamidine 0.1% and neomycin 1%.26 Since then, combination therapy has remained the mainstay of treatment, with application of two or more topical anti-amoebics throughout the day.
Effective cationic antiseptics, most notably polyhexamethylene biguanide (PHMB, 0.02%) and chlorhexidine (0.02%), have shown efficacy in both monotherapy and combination therapy with a diamidine26 such as Brolene (propamidine isethionate and dibromopropamidine isethionate).8 Cationic antiseptics are strong bases whose anti-amoebic potential stems from their ability to lyse cells by binding to the phospholipid bilayer and causing fatal membrane damage.27 Use of these antiseptics has brought large improvements among both non-contact lens and contact lens-wearing AK patients.8 Diamidines work by either interfering with methyl group transfer or directly disturbing amoebic nucleic acids.28 Hourly application of 0.1% propamidine isethionate and 0.15% dibromopropamidine has exhibited success only in the early stages of disease. Corticosteroids are also sometimes prescribed to reduce inflammation and pain, but can simultaneously increase the pathogenicity of the amoebae by suppressing the patient's immune response.29
Surgical treatment remains a necessary option when infection has progressed beyond the point of anti-infective viability. Penetrating keratoplasty is used to remove and replace infected tissue, and an antiseptic/ diamidine regimen is continued.
Acanthamoeba keratitis is a rare disease that, if undetected, can permanently damage the cornea and anterior tissues, leaving the patient blind in one or both eyes. The threat of AK should be considered when one makes the decision whether or not to use contact lenses as well as what type of lens to use. The importance of proper lens care cannot be overstated, as it's a sure-fire way to prevent not only Acanthamoeba infection, but a host of other unpleasant, and more common, infections. Community awareness of the dangers associated with dechlorination and the storage of stagnant water, more widespread adoption of proper contact lens habits, and knowledgeable diagnosis are the only ways to effectively treat Acanthamoeba and halt its surging prevalence.
Dr. Abelson, an associate clinical professor of ophthalmology at
1.
2. Meier PA, Mathers WD, Sutphin JE, Folberg R, Hwang T, Wenzel RP. An epidemic of presumed Acanthamoeba keratitis that followed regional flooding. Arch Ophthalmol 1998;116:8:1090-4.
3. Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 2007;50:1-26.
4. Thebpatiphat N, Hammersmith KM, Rocha FN, Rapuano CJ, Ayres BD, Laibson PR, Eagle RC, Cohen EJ. Acanthamoeba keratitis: A parasite on the rise. Cornea 2007;26:6:701-6.
5. Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 2003;16:2:273-307.
6. Mazur T, Hadás E, Iwanicka I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med and Parasitol 1995;46:2:106-8.
7. Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportnistic pathogens of humans and animals. Int J Parasitology 2004;34:9:1001-27.
8. Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol 2000;84:1103-8.
9. Stehr-Green JK, Bailey TM, Brandt FH, Carr JH, Bond WW, Visvesvara GS. Acanthamoeba keratitis in soft contact lens wearers. A case-control study. JAMA 1987;258:1:57-60.
10. Hiti K, Walochnik J, Haller-Schober EM, Faschinger C, Aspöck H. Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br J Ophthalmol 2002;86:2:144-6.
11. Shoff ME, Joslin CE, Tu EY, Kubatko L, Fuerst PA. Efficacy of contact lens systems against recent clinical and tap water Acanthamoeba isolates. Cornea 2008;27:6:713-9.
12. Joslin CE, Tu EY, McMahon TT, Passaro DJ, Stayner LT, Sugar J. Epidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreak. Am J Ophthalmol 2006;142:2:212-217.
13. Mathers W. Use of higher medication concentrations in the treatment of Acanthamoeba keratitis. Arch Ophthalmol 2006;124:6:923.
14. Sansanayudh W, Cevallos V, Porco TC, Margolis TP, Lietman TM, Acharya NR. Fusarium and Acanthamoeba keratitis: Can a single center detect outbreaks? Br J Ophthalmol 2008;95:5:720-1.
15. Joslin CE, Tu EY, Shoff ME, Booton GC, Fuerst PA, Mchahon TT, Anderson RJ, Dworkin MS, Sugar J, Davis FG, Stayner LT. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol 2007;144:2:169-180.
16. Butcko V, McMahon TT, Joslin CE, Jones L. Microbial keratitis and the role of rub and rinsing. Eye Contact Lens 2007;33:6:421-3.
17. Kilvington S, Gray T, Dart J, Morlet N, Beeching JR, Frazer DG, Matheson M. Acanthamoeba keratitis: The role of domestic tap water in the United Kingdom. IOVS 2004;45:1:165-169.
18.Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis 2007;20:2:129-41.
19.Alizadeth H, Neelam S, Hurt M, Niederkorn JY. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect Immun 2005;73:2:1061-8.
20.Watt K, Swarbrick HA. Microbial keratitis in overnight orthokeratology: Review of the first 50 cases. Eye Contact Lens 2005;31:5:201-8.
21. Hammersmith KM. Diagnosis and management of Acanthamoeba keratitis. Curr Opin Ophthalmol 2006;17:4:327-31.
22. Lam DS, Houang E, Fan DS, Lyon D, Seal D, Wong E;
23. Seal DV. Acanthamoeba keratitis update – incidence, molecular epidemiology and new drugs for treatment. Eye 2003;17:8:893-905.
24. Tu EY, Joslin CE, Sugar J, Booton GC, Shoff ME, Fuerst PA. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea 2008;27:7:764-72.
25. Gupta N, Tandom R. Investigative modalities in infectious keratitis. Indian Journal of Ophthalmology 2008;56:3:209-1.3
26. Wright P, Warhurst D, Jones BR. Acanthamoeba keratitis successfully treated medically. Br J Ophthalmol 1985;69:778–782.
27. Lim N, Goh D, Bunce C, Xing W, Fraenkel G, Poole TRG, Ficker L. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Amer J Ophthalmol 2008;145:130.
28. Wysenbeek YS, Blank-Porat D, Harizman N, Wygnanski-Jaffe T, Keller N, Avni I. The reculture technique: Individualizing the treatment of acanthamoeba keratitis. Cornea 2000;19:4:464-7.
29. Berger ST, Mondino BJ, Hoft RH, Donzis PB, Holland GN, Farley MK, Levenson JE, Successful medical management of Acanthamoeba keratitis. Amer J Ophthalmol 1990;110:4:395–403.