Until recently, there was a mystery at the center of the glucocorticoid anti-inflammatory mechanism. It was well-known that glucocorticoids entered the cell nucleus bound to cytoplasmic glucocorticoid receptors. But what happened inside the nucleus? On the eve of the 21st century, breakthrough research found new roles for molecules known as histone acetylizing transcriptional coactivators.1 It's now possible for researchers to look through the nuclear envelope into hidden areas of the steroidal mechanism. In this article, we'll explain the science behind histone acetylizing transcriptional coactivators, and discuss the implications they hold for medicine in general and ophthalmology in particular.
Histone Acetylation
DNA is essentially a very long, very thin thread of genetic information. There are 2 to 3 meters of DNA in every cell of the human body.2 In order to fit all of this DNA into the tiny nucleus of the eukaryotic cell, DNA is neatly, tightly coiled around a protein core. This DNA and protein complex, called the chromatin, has important roles in mitosis and meiosis, but in this article we'll focus on chromatin's part in gene transcription.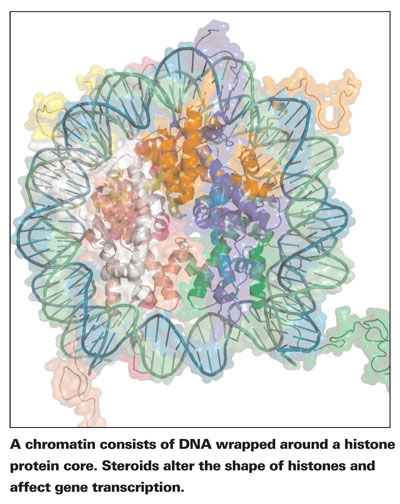
Molecules called histones make up the protein backbone of the chromatin complex. The shape of histones directly affects gene transcription.
The enzyme RNA polymerase II (pol II), which transcribes DNA and starts the process of protein synthesis, is a large molecule (550 kDa).2 Pol II cannot squeeze between the DNA coils when DNA is tightly wrapped around histones. DNA must be slightly unraveled for genes to be transcribed. Since histones control the tension of DNA threads, they effectively control the expression of genes. Histones can therefore be thought of as the gatekeepers of the DNA; certain genes cannot be translated without prior chromatin histone modification.1
The shape of histones can be altered by molecules with histone acetylative properties. Histone acetyltransferases (HATs) loosen the histone grip on DNA by adding acetyl functional groups to the histones. Histone deacetylases (HDACs) have the opposite effect; they retighten DNA around histones, by removing acetyl functional groups.3
In terms of gene transcription, HATs allow genes to become expressed, and HDACs repress genes.
In 2001, a review article by Fyodor Urnov, PhD, and Alan Wolffe, PhD, brought histone acetylation research tantalizingly close to the steroidal mechanism. Transcriptional coactivators such as cAMP response element binding proteins (CBP) and p300/CBP-associated factors have latent HAT effects, Drs. Urnov and Wolffe explained. Some proteins, in other words, can loosen the histone grip on DNA, and mediate transcription of exposed DNA too. Research into gene transcription has recently shown that HATs and HDACs are crucial in the intra-nuclear, anti-inflammatory mechanism of glucocorticoids.4
Inflammatory Gene Repression
When a cell is injured, nuclear factor kappa B (NF-kB) acts like a chemical "911 call." NF-kB enters the nucleus, the "headquarters" of the cell, where it recruits CBP or p300/CBP-associated factors.5 As Drs. Urnov and Wolffe described, CBP and p300/CBP-associated factors have HAT effects. HAT activity loosens DNA, allowing RNA polymerase II to transcribe inflammatory genes.6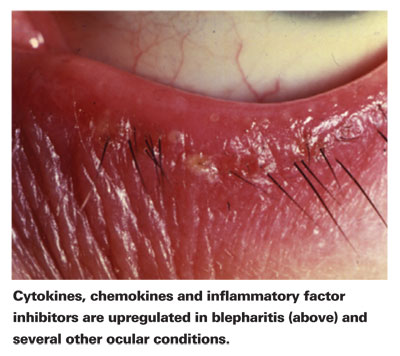
Steroids disrupt the transcription of inflammatory genes in two different ways: by inhibiting HATs and promoting HDACs. Glucocorticoids enter the cell's nucleus bound to glucocorticoid receptors (GRs) and disrupt the HAT activity that's taking place inside. At the same time, glucocorticoids bound to receptors also recruit HDACs. The HATs stop loosening DNA, and HDACs pull the DNA tight against the histones. RNA polymerase doesn't have a chance to reach the inflammatory genes on the DNA, which, if activated, spark a cascade of inflammatory responses.
Anti-inflammatory Gene Expression
Steroids don't just stop the transcription of inflammatory genes, they also promote the transcription of anti-inflammatory genes. After entering the nucleus bound to GRs, steroids recruit transcriptional factors such as CBP and SRC-1, which have HAT characteristics as Drs. Urnov and Wolffe described.4 The HATs work with other molecules to unwind DNA and transcribe anti-inflammatory genes. (While anti-inflammatory genes are significant, the anti-inflammatory effect of steroids observed clinically is primarily due to the repression of inflammatory genes.4)
Steroids' Advantage
Corticosteroids are potent, in part, because they go directly to the nucleus of the cell. By decreasing the transcription of inflammatory genes, steroids are able to slow the production of inflammatory cytokines, chemokines, inducible enzymes, endothelin-1 receptors and intracellular adhesion molecules.
Some of the following molecules are inhibited by steroids on the transcriptional level and are famously implicated in the pathogenesis of the inflammatory ocular conditions:
• interleukin-2, -4, -6 and -8;
• cyclooxygenase-2 (COX-2);
• phospholipase A2;
• tumor necrosis factor (TNF)-a;
• monocyte chemoattractant protein-1, -3 and -4; and
• intracellular cell adhesion molecule-1.4
By increasing the transcription of anti-inflammatory genes, steroids are able to accelerate the production of inflammatory inhibitory molecules including lipocortin-1, annexin-1 and clara cell protein 10, all of which are phospholipase A2 inhibitors, and IkB, an NF-kB inhibitor.4 Steroids are, in other words, able to affect the concentrations of dozens of molecules involved in the inflammatory process by going to the source: the DNA.
The aforementioned cytokines, chemokines and inflammatory factor inhibitors affect organs outside of the eyes including the lungs (asthma), the appendix (appendicitis), the brain (meningitis) and the skin (dermatitis). But they are also upregulated in a broad spectrum of inflammatory ocular conditions, including allergic conjunctivitis, blepharitis, uveitis, endophthalmitis and cystoid macular edema.7
HAT and HDAC steroidal transcription pathways are powerfully anti-inflammatory in the eye and elsewhere in the body because they stop inflammatory molecules before they are synthesized or even transcribed.
Steroids are more potent than non-steroidal anti-inflammatory drugs such as COX inhibitors because they act earlier in the inflammatory innate immune response. Steroids inhibit inflammation before phospholipase A2 can continue along to the COX pathway, synthesizing prostaglandins, and before it can continue along the lipoxygenase pathway, synthesizing leukotrienes.1 COX inhibitors, by contrast, disrupt the COX pathway later, after phospholipase A2 has been synthesized.
A Novel Therapy on the Horizon?
Research has recently discovered steroidal HAT and HDAC pathways which affect gene transcription, and these pathways are being marked as targets for novel therapies. Researchers in the
The HDAC steroidal pathway may also have therapeutic potential. The respiratory drug theophylline, a proven anti-inflammatory molecule naturally found in teas, has been shown to recruit HDACs.9 In an article discussing respiratory inflammation, researchers hypothesized that a novel NSAID treatment mimicking steroidal HAT or HDAC pathways could be therapeutically useful.4
The clinical utility of steroids is limited by the concerning ocular and systemic complications that are associated with long-term steroid treatment. In the eye, exposure to steroids has been linked to posterior subcapsular cataract formation, and increased intraocular pressure.7
Topical ocular steroids are readily absorbed systemically, where they can cause musculoskeletal, gastrointestinal, nervous, cardiovascular, metabolic or endocrine disorders.7 A novel anti-inflammatory drug following the corticosteroid mechanism of action inside the nucleus could theoretically duplicate the anti-inflammatory potency of steroids while avoiding some of their deleterious side effects.
The clinical utility of steroids was first demonstrated in 1949 by Nobel laureates Philip Hench, MD, and Edward Kendall, PhD.7 Within a year, steroids were being used in ophthalmology.7 Understanding of the corticosteroid mechanism of action has evolved more slowly.
Recent research in chromatin remodeling and gene transcription is filling in some major gaps in our knowledge of the steroid anti-inflammatory mechanism. Some of the newly clarified steroidal pathways may even eventually be targeted by novel therapies. For now, however, HAT and HDAC pathways help explain the value of an essential ophthalmic drug: the ocular steroid.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Urnov FD, Wolffe AP. Chromatin remodeling
and transcriptional activation: The cast (in order of appearance). Oncogene 2001;20:2991-3006.
2. McGraw Hill Encyclopedia of Science and Technology.
3. De Ruijter AJ, Van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem Cell Biol 2001;79:337-348.
4. Adcock IM, Ito K, Barnes PJ. Glucocorticoids: Effects on gene transcription. Proc Am Thorac Soc 2004;1:247-54.
5.
6. Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1‚ induced histone H4
acetylation on lysines 8 and 12. Mol Cell Biol 2000;20:6891-6903.
7. Albert DM. Jakobiec FA, eds. Principles and Practice of Ophthalmology. W.B. Saunders Publishing:
8. Turlais F, Hardcastle A, Rowlands M, et al. High throughput screening for identification of small molecule inhibitors of histone acetyltransferases using scintillation microplates. Anal Biochem 2001;298:62-8.
9. Ito K, Lim S, Caramori G, et al. A molecular mechanism of action of theophylline:
Induction of histone deacetylase activity to decrease inflammatory gene expression. PNAS 2002;99:8921-8926.