Presentation
A 68-year-old African-American male presented to Wills Eye Hospital for evaluation of distorted central vision in his right eye in the setting of persistent uveitis. Seven months prior, he had undergone pars plana vitrectomy to remove retained lens material following cataract surgery. His postoperative course was complicated by uveitis in his right eye, which was controlled with once daily difluprenate 0.05% dosing, but flared with an attempt to taper further. Notably, he had a remote history of bilateral sarcoid-associated uveitis.
Medical History
In addition to the vitrectomy mentioned above, his ocular history was significant for cataract extraction with a Crystalens AO (Bausch + Lomb) posterior chamber lens in both eyes approximately one year prior to presentation, primary open angle glaucoma that was being medically managed, chronic right eyelid ptosis and prior bilateral uveitis attributed to sarcoidosis.
 |
Past medical history included sarcoidosis, diagnosed by cervical lymph node biopsy, with no systemic symptoms for the past six years off oral prednisone. He also had a history of hypertension and atrial fibrillation, treated with anticoagulation.
Family history was significant for glaucoma in his mother. The patient was a nonsmoker, and other social history was noncontributory. His ocular medications included difluprednate 0.05% once daily OD and brimonidine 0.2% b.i.d. OU. His oral medications included: apixaban 5 mg daily; metoprolol 50 mg b.i.d.; atorvastatin 20 mg daily; and diltiazem 120 mg daily.
Examination
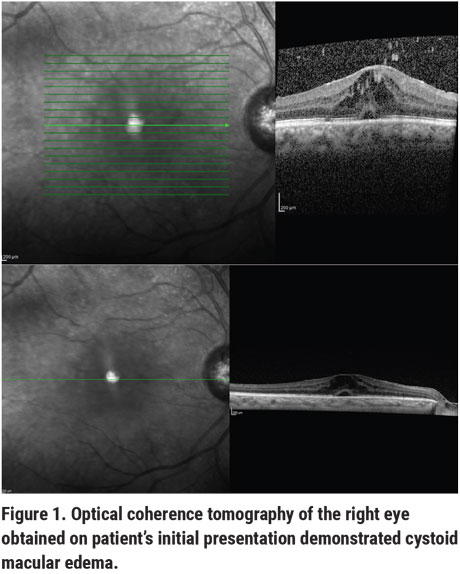
Ocular examination demonstrated a best-corrected visual acuity of 20/100-2 OD and 20/30 PH 20/25-1 OS. Intraocular pressures were 18 mmHg OD and 12 mmHg OS by Tonopen. Pupillary exam revealed equal, round and brisk pupils OU without a relative afferent pupillary defect. Confrontation visual fields were full OU. Anterior slit lamp examination of the right eye showed a well-healed phacoemulsification incision and stable arcuate incisions, scattered pigmented endothelial deposits but no active keratic precipitates, a deep and quiet anterior chamber and a Crystalens in the capsular bag superiorly and in the sulcus inferiorly without vitreous prolapse. Anterior slit lamp examination of the left eye showed a well-healed phacoemulsification scar, stable arcuate incisions, a Crystalens centered in the capsular bag and no other abnormalities. Fundoscopic examination showed a cup-to-disc ratio of 0.5 in the right eye and 0.65 in the left eye. Other notable findings in the right eye were asteroid-like opacities with rare cells in the vitreous and macular edema without evidence of vasculitis, snowballing or scleritis. Optical coherence tomography demonstrated retinal thickening consistent with macular edema (Figure 1).
What's your diagnosis? What further workup would you pursue? The diagnosis appears below.
Diagnosis and Management
This patient’s history, examination, and optical coherence tomography findings were consistent with cystoid macular edema associated with pseudophakia and uveitis. He had multiple risk factors for CME including prior cataract extraction complicated by retained lens material, vitrectomy, uveitis and sarcoidosis. Notably, he didn’t have diabetes, wasn’t taking prostaglandin eye drops for his glaucoma and didn’t have any systemic sarcoidosis symptoms.
While his diagnosis of CME was confirmed with OCT, the subsequent management of his CME was challenging, and his course was atypical. Initial treatment of his CME at Wills included three doses of periocular sub-Tenon’s triamcinolone acetonide and one injection of intravitreal bevacizumab over nine months, with continuation of his daily difluprednate 0.05%. His CME persisted without significant improvement despite these interventions, lack of systemic sarcoidosis symptoms and ongoing suppression of his uveitis. Over the following year, his severe CME was resistant to oral acetazolamide (initially 125 mg t.i.d., then increased to 250 mg t.i.d.), two injections of intravitreal triamcinolone acetonide and three injections of aflibercept. After two years of recalcitrant CME, there were low expectations for improvement, and he was started on ketorolac 0.5% q.i.d. and continued on difluprednate 0.05% once daily.
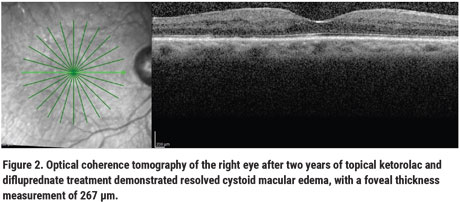
Surprisingly, after two years of topical ketorolac and difluprednate treatment, his CME resolved on OCT (Figure 2), with a foveal thickness measurement of 267 µm, and visual acuity improved to 20/60. His treatment course was complicated by one episode of ocular hypertension (maximum 30 mmHg) 21 months after his last aflibercept injection, which improved with timolol.
 |
Examination
Cystoid macular edema, which presents as distorted central vision and macular thickening, can result from a diverse range of pathologies, including uveitis, postoperative inflammation, diabetes, retinitis pigmentosa, retinal vein occlusions and others. CME occurs when extracellular fluid accumulates in the outer plexiform and inner nuclear retinal layers and forms cystic collections between retinal septa.1,2 Different diseases may lead to CME through disparate mechanisms, so determining the underlying etiology of CME is important for selecting an appropriate treatment. Although CME is often considered as a single disease entity, it is in fact a complex pathology with multiple pathways leading to its development, many of which are still not understood.3
This discussion focuses on two types of inflammatory CME (uveitic and pseudophakic), because these are most relevant to our patient’s case. Approximately 40 percent of uveitis patients develop macular edema, 25 percent of which is CME, and macular edema is the primary cause of vision loss in uveitis.2,4–7 Risk factors for developing CME included older age at onset of uveitis, and chronic and persistent uveitis.6 Pseudophakic CME complicates 0.2 to 3.3 percent of cataract extractions and typically occurs one to six weeks after surgery.8 Risk factors include postoperative inflammation, combined procedures such as phacovitrectomy, and complications such as retained lens fragments.8
In both uveitic and pseudophakic CME, inflammatory cytokines and vascular endothelial growth factor are thought to cause disruption of the blood retinal barrier, which is composed of tight junctions between retinal pigment epithelial cells and capillary endothelial cells.3,9 Treatments for uveitic and pseudophakic CME focus on reducing inflammatory cascades and VEGF, and targeting retinal fluid flow.
Steroids, in various forms, are the mainstay of inflammatory CME management.7 Regional steroids, in particular, play a crucial role in treating eyes with suppressed uveitis but ongoing macular edema.7,10 A recent randomized clinical trial compared the efficacy and safety of the three most commonly used regional steroid therapies for uveitic macular edema, including periocular triamcinolone acetonide, intravitreal triamcinolone acetonide and the intravitreal dexamethasone implant. Results published in 2019 showed that all three treatment groups had improved central macular subfield thickness on OCT, but the two intravitreal treatment groups had greater and faster onset of efficacy than the periocular group. Intravitreal injections, however, carried a slightly higher risk of IOP elevations than periocular treatment.7
When CME is resistant to steroids, anti-VEGF injections may be used. They have been shown to temporarily decrease macular thickness in persistent inflammatory CME in prior studies—but only if the uveitis is suppressed.11,12 However, there has also been a report of a 1.3-percent incidence of acute intraocular inflammation following anti-VEGF injections.13 Carbonic anhydrase inhibitors are also thought to be effective in steroid-refractory CME cases because they work on RPE dysfunction, which anti-inflammatory treatments don’t target.14 One study showed that 68 percent of patients with chronic inflammatory CME had improvement or resolution in their central macular subfield thickness on OCT after acetazolamide was added to their treatment regimens.14 A benefit of acetazolamide is that it doesn’t carry the risk of elevated intraocular pressure, but it can result in numerous systemic side effects with long term use.14
When our patient’s CME failed to respond to steroids, anti-VEGF agents and acetazolamide, he was started on a topical NSAID. In the treatment algorithm for pseudophakic CME, NSAIDs are considered a first-line intervention with concurrent topical steroids.8,9 They act by inhibiting cyclooxygenase enzymes, which are downstream of arachidonic acid released by uveal tissues postoperatively. A prior study showed that 54 percent of patients with pseudophakic CME had complete resolution of edema on OCT with six weeks of topical steroid and nepafenac treatment.15 However, these results may be confounded by pseudophakic CME’s typical course, which tends to improve spontaneously.14 Compared to steroids, NSAIDs have a less extensive anti-inflammatory effect, so they are typically not expected to improve steroid-resistant CME.8
After two years of treatment with topical NSAIDs and difluprednate, our patient’s CME resolved. This atypical clinical response demonstrates that although CME may have a common clinical presentation, it’s modulated by diverse mechanisms yet to be fully characterized and which may respond to unexpected treatment modalities. Therefore, despite the availability of multiple evidence-based treatments for inflammatory CME, its management continues to be challenging. REVIEW
1. Fardeau C, Champion E, Massamba N, et al. Uveitic macular edema. Eye 2016;30:1277–1292.
2. Tomkins-Netzer O, Lightman S, Drye L, et al. Outcome of treatment of uveitic macular edema: The Multicenter Uveitis Steroid Treatment trial 2-year results. Ophthalmology 2015;122:2351–2359.
3. Daruich A, Matet A, Moulin A, et al. Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res 2018;63:20–68.
4. Lardenoye CWTA, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology 2006;113:1446–1449.
5. Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–336.
6. Grajewski RS, Boelke AC, Adler W, et al. Spectral-domain optical coherence tomography findings of the macula in 500 consecutive patients with uveitis. Eye 2016;30:1415–1423.
7. The Multicenter Uveitis Steroid Treatment Trial (MUST) Research Group, Thorne JE, Sugar EA, et al. Periocular triamcinolone versus intravitreal triamcinolone versus intravitreal dexamethasone implant for the treatment of uveitic macular edema: The PeriOcular versus INTravitreal corticosteroids for uveitic macular edema (POINT) Trial. Ophthalmology 2019;126:283.
8. Grzybowski A, Kanclerz P. The role of steroids and NSAIDs in prevention and treatment of postsurgical cystoid macular edema. Curr Pharm Des 2018;24:4896–4902.
9. Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin 2010;50:139–153.
10. Leder HA, Jabs DA, Galor A, et al. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol 2011;152:441–448.e2.
11. Mackensen F, Heinz C, Becker MD, et al. Intravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: A pilot study. Retina 2008;28:41–45.
12. Bae JH, Lee CS, Lee SC. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina 2011;31:1:111-8.
13. Johnson D, Hollands H, Hollands S, et al. Incidence and characteristics of acute intraocular inflammation after intravitreal injection of bevacizumab: A retrospective cohort study [Internet]. Can J Ophthalmol 2010;45:3:239-42.
14. Pepple KL, Nguyen MH, Pakzad-Vaezi K, et al. Response of inflammatory cystoid macular edema to treatment using oral acetazolamide. Retina 2019;39:948–955.
15. Sengupta S, Vasavada D, Pan U, et al. Factors predicting response of pseudophakic cystoid macular edema to topical steroids and nepafenac. Indian J Ophthalmol 2018;66:827–830.



