Now, thanks to advances in technology such as dyes and imaging, that include optical coherence tomography, we’re beginning to sort out how best to make this approach work. Hopefully, we’ll soon be able to determine which eyes are good or poor candidates for trabecular bypass. As we learn more about how the outflow system works and how to repair it when it malfunctions, we’re beginning to get a sense of the place trabecular bypass should hold in glaucoma treatment, and how we can best use it to care for our patients.
Here, I’d like to talk about some of what we’ve learned in this area and where it might lead us. In particular, I’d like to challenge some key popular ideas about what constitutes “successfully” treating glaucoma—ideas that may be preventing many of us from offering patients effective treatment options that are far more patient-friendly than a trabeculectomy or tube shunt.
Placing Stents Effectively
Many of the so-called micro-invasive glaucoma surgeries, or MIGS, offer minimally invasive ways to bypass the trabecular meshwork. I was involved in the first study of the iStent (Glaukos), which was also the first study ever to test so small a device. We wanted to find out how much pressure reduction we could achieve by using a device to get into the canal of Schlemm and bypass the trabecular meshwork. Unfortunately, there was a lot we didn’t know about the device and how to use it most effectively. Also, we were focused on meeting the requirements and expectations of the U.S. Food and Drug Administration, and we were restricted to implanting the device during cataract surgery. When designing the trial we had not refined some important things, such as washout periods, control groups, adding medications during the trial and other factors.
The result was that the data from the iStent trial was kind of messy. Overall, the data seemed to indicate that there wasn’t a big difference between phaco alone and the iStent combined with phaco. At the time, I told the company that those results didn’t make sense to me because I had some patients who got terrific results—as much as a 35-percent reduction in IOP. Of course, these were the early days; there was no agreement about technique, or where to put the stent, or how to determine if it was even in the canal.
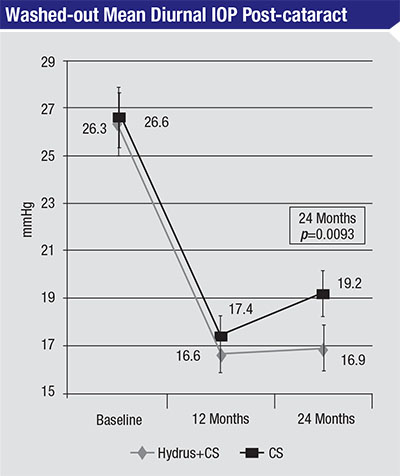 |
| In this 2015 study, 100 eyes with open-angle glaucoma and cataract and a washed-out diurnal intraocular pressure between 21 and 36 mmHg underwent cataract surgery, with or without implanting a Hydrus Microstent. At 24 months, washed-out mean diurnal IOP was significantly lower in the group that received the Hydrus implant. There were no differences in follow-up visual acuity between the groups.3 |
I was pretty obsessive about making sure the stent was actually in the canal. I would look for aqueous veins, and we knew from histopathological studies that there is a larger abundance of collector channels inferonasally, so I tried to target the inferonasal area. After I inserted the stent I would look at it under high magnification and check for blood reflux. Perhaps not surprisingly, a subset of patients in the trial looked really good. During the trial I did get to observe a few other surgeons who were participating, and I saw one surgeon who failed to get the stent into the canal at all. That data is part of the study as well.
Today, we’ve learned a lot more about the trabecular meshwork, the canal and the collector channels, and the mixed results from that trial are beginning to make sense. For one thing, because outflow is segmental, we now know that it’s beneficial to implant the device close to a patent collector channel. Recently, other studies have confirmed this. Reay Brown, MD, and his colleagues published a paper last year involving 47 eyes, in which they validated that the stents were placed effectively;1 they reported a mean IOP reduction of 3.2 mmHg, which was statistically significant and much better pressure control than we achieved in the first iStent study. So it’s clear that targeted placement makes a big difference in the outcome.
Their study’s findings highlight the importance of correctly identifying the canal (to ensure that the device is actually in the canal) and then either being able to identify the collector channels and their condition, so the correctly implanted device has a chance to work, or using multiple stents and hoping that one (or both) are close to a functioning collector channel. Technology like OCT is beginning to make targeted placement possible, thanks to improved visualization of these structures.
Because placing the stent close to a functioning collector channel can be challenging, another way to increase the odds of effective pressure lowering is to implant more than one stent. That’s the idea behind the iStent Inject Trabecular Micro-Bypass Stent, which comes preloaded with two stents. Results from a clinical trial of that device are indeed showing better pressure reduction than is seen in patients who receive a single iStent. A 17.5-percent between-group treatment difference in favor of the iStent Inject group was statistically significant (p=0.02) at the >50 per-cent level of IOP reduction.2 It seems that implanting more stents does a better job of tapping into Schlemm’s canal and gets us closer to the areas where outflow is functioning. In my experience, using two iStents—or a single well-placed iStent—gives us pressures somewhere in the mid to upper teens.
The importance of access to patent collector channels is also supported by the work being done with the Hydrus Micro-stent (Ivantis), which dilates and scaffolds a section of the canal. Trials are showing pretty good pressure reduction with the Hydrus, similar to what we’re seeing with two iStents.3 (See chart)
“MIGS and Meds”
That brings us to the issue of what constitutes a “successful” glaucoma treatment. Many ophthal-mologists believe that ideally we should get our patients completely off of medications. Given that the target pressure for many patients with significant glaucoma is 10 to 14 mmHg, the trabecular bypass approach is seen as a failure because it can’t get most patients to that level by itself.
I believe that thinking needs to change, because the alternatives we have to offer these patients are problematic. The issues accompanying trabeculectomy and tube shunts are well-known. At the same time, implanting one or two well-placed iStents or a Hydrus, and adding a single medication, can easily get most patients into the 10- to 14-mmHg range. The end result is that you’ve done a minimally invasive surgery and the patient has to use one drop a day, and you’ve achieved a result equal to what you would have achieved with a trabeculectomy.
Make no mistake: Patients are happier with an iStent than they are after a trabeculectomy or tube shunt. I often have newer patients ask for the iStent because they’ve spoken to others who have had a trabeculectomy or tube shunt and they didn’t like what they heard. In addition to being an associate professor at Johns Hopkins University in Baltimore, I’m chief of glaucoma at King Khaled Eye Specialist Hospital in Riyadh, Saudi Arabia, and I’ve found that globally, the acceptance of the iStent is much higher than trabeculectomy. Combining a MIGS surgery like the iStent or Hydrus with a single medication may achieve the same level of pressure reduction with far less irritation for the patient.
This is why I came up with the phrase “MIGS and meds.” It reflects the idea that this combination has some appeal. In the past, MIGS has been unfairly cast as an inferior option for patients, simply because they have to continue using one drop a day. (In reality, quite a few patients still end up using a drop every day after a trabeculectomy or tube shunt, too.) Reaching the target pressure with a minimally invasive surgery and one drop a day is a better success, in my opinion, than a trabeculectomy. At the least, it’s reasonable to start with this and move to the more aggressive surgeries if this method fails. Patients are much more comfortable, and complications are much less likely.
Seeing the Unseen
Of course, there are patients for whom trabecular bypass isn’t a good option. If the patient has raised episcleral venous pressure or atrophied collector systems, then repairing or improving the trabecular bypass system probably won’t lower intraocular pressure much, if at all (and of course, certain types of glaucoma, such as neovascular glaucoma, wouldn’t be helped by this approach.) The problem is, we don’t have any tools right now that can do a good job of measuring either one of those things. So for now when a patient needs surgery I first opt to try implanting an iStent—or two of them—and see if it works. If it doesn’t, then I move on to other options, because the failure suggests that the episcleral venous pressure is high and/or the collector system is not functioning.
Meanwhile, with the steady im-provement in technologies like OCT and dyes, we may soon be able to look at the canal and identify which patients will be good or poor candidates for trabecular bypass. At this point, the latest OCT technology can reveal which areas of the canal are more dilated and more collapsed, and we can sometimes see collector channels. What this means is still unclear; however, thanks to the work of Murray Johnstone, MD, we know that the canal of Schlemm is a dynamic entity that functions as a biomechanical pump. In a healthy eye, blinking or moving the eye causes the trabecular meshwork to move and push aqueous out. Since the current OCT images we have are snapshots, it’s possible that the collapsed areas of the canal are actually the sections that are functioning; the image may have caught the canal as it has pushed some aqueous into the collector channels. As time goes on, we’ll find out. (Note: The OCT we’ve been using for this purpose is not yet FDA-approved.)
We’re in the infancy of being able to do this, but it seems clear that as our ability to see these details and our understanding of what they are telling us improves, we’re going to start getting answers about who would be a good trabecular bypass candidate.
The Cataract Factor
Another factor that has impacted how surgeons see MIGS options like the iStent is another legacy of the original trial of the iStent: the idea that this surgery makes the most sense when it’s combined with cataract surgery. (The FDA approval for that circumstance alone, of course, has limited reimbursement as well.)
I’ve been doing a lot of Hydrus and iStent implantations in the Middle East as standalone procedures—no cataract surgery involved—and I can testify that it works in either pseudophakic or phakic eyes. Unfortunately, we have limited clinical data on the iStent as a standalone procedure outside of cataract surgery, although some studies do support this premise. There is currently a clinical trial evaluating the effectiveness of the Hydrus without cataract surgery. Also, a study conducted in Armenia, where patients received two iStents and a single medication, showed that all 39 patients achieved an IOP reduction of at least 20 percent and had an IOP of 14 mmHg or less at 18 months.4 So, I believe cataract surgery is a secondary issue. If our perception of MIGS as not being “successful” for many patients were to shift, this might be more obvious.
Finding the Best Option
I think the biggest hurdle with the idea of trabecular bypass and devices like the iStent is that so many surgeons accept the idea that it doesn’t work. They may look at the first paper and take that data as evidence that this conclusion is justified. But in reality, trabecular bypass with the iStent works very well in many patients. If some of those patients need to use one drop a day to reach our target, that’s a pretty small burden to bear compared to the side effects of a trabeculectomy or tube shunt. My patients who have had an implant are much happier than my patients who have had trabeculectomies and tubes.
As our ability to judge which patients are most likely to benefit from trabecular bypass improves, success rates should increase. Nevertheless, the ultimate popularity of this approach may depend on another factor: other MIGS devices in the pipeline. Some of these are aiming to lower pressure by siphoning aqueous into the suprachoroidal space. Early data does indicate that this approach can work well; data from the trial of the CyPass device (Alcon) is significantly better than the data from the first iStent trial (article in press). The safety data has also been very strong for the suprachoroidal devices that are in the pipeline; they seem to be well-tolerated without any significant issues beyond a little bit of bleeding, which is usually transient. There’s been almost no hypotony. So these will probably be good options as well.
Which approach ends up being more popular may depend on timing; if it’s still unclear which patients are good candidates for trabecular bypass when a suprachoroidal device is approved, surgeons may be inclined to move in that direction. On the other hand, my personal belief is that taking advantage of the physiological outflow pathway through the trabecular meshwork makes the most sense. With the possible addition of a single medication, I think it’s a great first-line surgical procedure, at least in patients for whom I think it will work.
In order for “MIGS and meds” to work in the long run, we will definitely need to become better at visualizing the canal and collector channels so we can readily identify patients who are the best candidates. In the meantime, using more than one stent, or a new device like the Hydrus, will help surgeons ensure that bypassing the trabecular meshwork provides as much pressure relief as possible. With the addition of a single medication, even challenging patients should be able to reach our target pressures. REVIEW
In my experience, patients who have gone down this path are far happier than those with trabeculectomies or tube shunts—and that’s not a bad definition of success.
Dr. Craven is an associate professor at Johns Hopkins University and chief of glaucoma at King Khaled Eye Specialist Hospital in Riyadh, Saudi Arabia. He is a consultant to Allergan, Alcon, Aerie, Transcend and Ivantis.
1. Brown RH, Gibson Z, Zhong L, Lynch MG. Intraocular pressure reduction after cataract surgery with implantation of a trabecular microbypass device. J Cataract Refract Surg 2015;41:6:1318-9.
2. Fea AM, Belda JI, Rekas M, Junemann A, Chang L, Pablo L, et al. Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol 2014;8:875-82.
3. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, Larrosa JM, Fea A, Lemij H, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 2015;122:7:1283-93.
4. Ahmed, II, Katz LJ, Chang DF, Donnenfeld ED, Solomon KD, Voskanyan L, et al. Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma. J Cataract Refract Surg 2014;40:8:1295-300.



