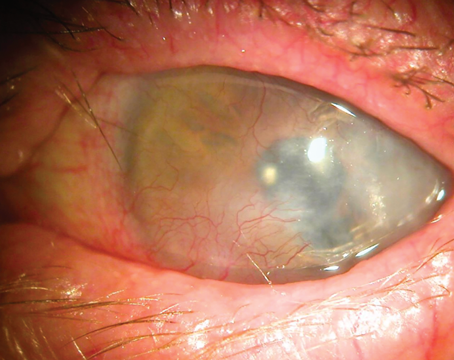
According to the 2023 U.S. Eye Banking Statistical Report, the domestic use of DALK tissue has been declining.1 While penetrating keratoplasty, DSAEK and DMEK employ the lion’s share of corneal tissue—28.9 percent, 32.4 percent and 34.2 percent, respectively—anterior lamellar keratoplasty represents just 1.2 percent of intermediate-term corneas.1 Experts say there are a number of reasons for this, from the procedure’s technical difficulty and availability of scleral lenses and cross-linking as early alternatives, to the good visual outcomes and relative ease of penetrating keratoplasty. Nevertheless, DALK is still a valuable procedure to learn, as it preserves the host endothelium and offers a number of safety advantages and good optical quality.
Here, corneal specialists share pearls for performing DALK, when to convert to a full-thickness transplant and the postoperative complications to watch out for.
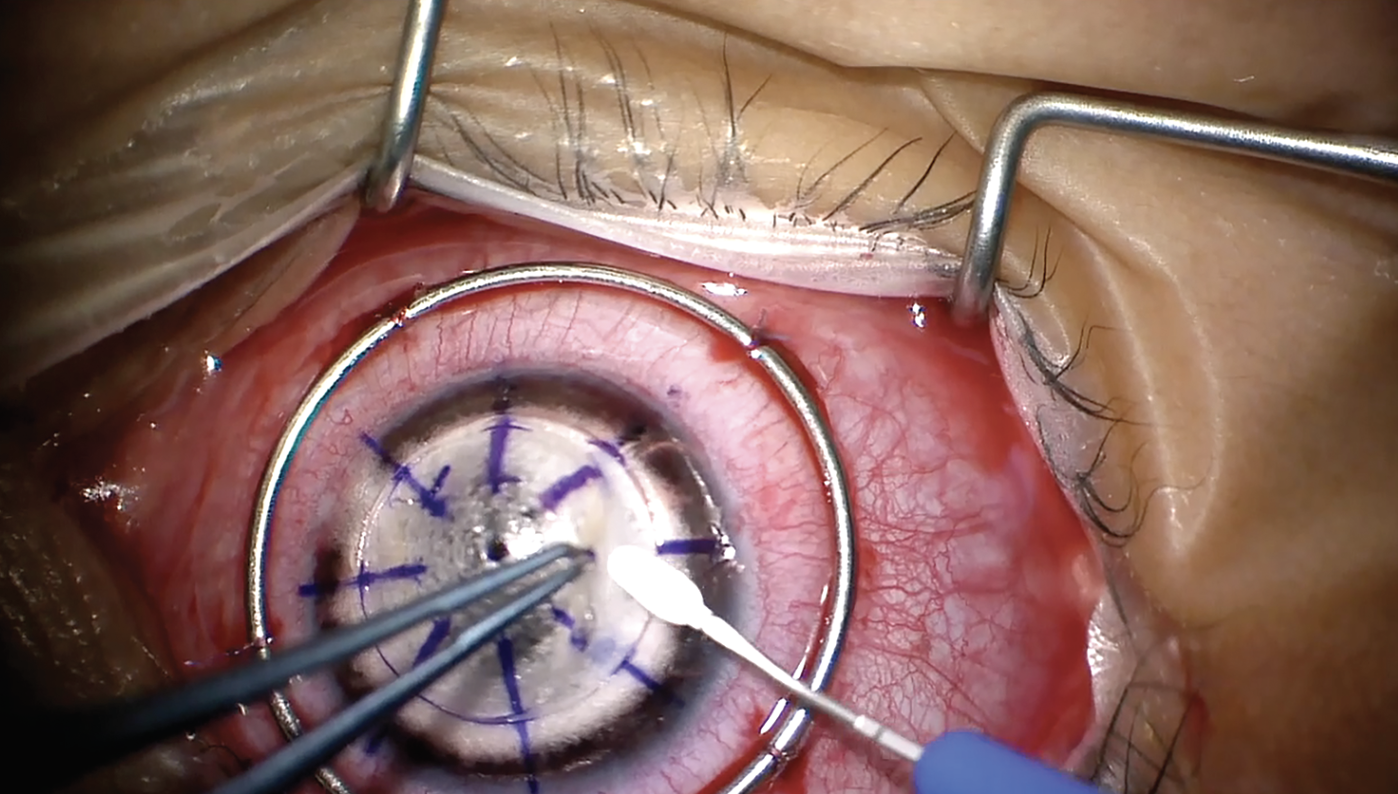 |
| Lamellar dissection is performed to debulk the cornea. All images: Farida E. Hakim, MD, and Gaurav Prakash, MD. |
Starting Out with DALK
The major advantage of performing DALK when indicated is the extremely low risk of graft rejection, according to Bennie H. Jeng, MD, chair of the Department of Ophthalmology and director of the Scheie Eye Institute at the University of Pennsylvania School of Medicine in Philadelphia. “You certainly eliminate all endothelial rejection, which is most cases of rejection since you’re not replacing the endothelial cells,” he says. “[DALK] is also safer [than PK] because it’s an outside-of-the-eye procedure, if done successfully, so there’s less risk of intraocular infection.”
Since DALK involves replacing the stroma and superficial layers of the cornea, this procedure is suitable for any pathology anterior to Descemet’s membrane provided the patient possesses a functional endothelium. Classic indications include keratoconus, corneal dystrophies and corneal scars. Eyes with endothelial disease such as Fuchs’, full-thickness infection healed by scarring or non-functional endothelium aren’t good candidates for DALK.
“DALK isn’t that commonly performed nowadays because of the advent of scleral contact lenses for keratoconus, and the indications for it are much later [in the disease],” says Gaurav Prakash, MD, an assistant professor of ophthalmology at the University of Pittsburgh School of Medicine. “So, doing a DALK cleanly is definitely more challenging now compared to when we didn’t have good quality scleral lenses or cross-linking and patients were sent for DALK earlier. All of these patients today come in with more advanced disease and are more challenging.
“The way to start doing DALK is to try to attempt a bubble in all the patients you’re doing a penetrating keratoplasty,” Dr. Prakash says. “Let’s say you have a patient with a corneal scar, and this patient has to undergo PK. That’s the best patient to attempt a big bubble in and see how your syringe is moving, how much air you’re injecting and getting the feel of how you’re going to get a bubble because eventually, you have to remove the endothelium in these patients, so the risk isn’t there. It’s definitely a more challenging procedure compared to normal LK, but the visual results are much more gratifying. This is what I recommend to new surgeons.”
Pre-Descemet’s Layer
Pre-Descemet’s layer, first described by Professor Harminder Dua and colleagues in 2013, is a layer of stroma anterior to Descemet’s membrane that provides a border to a cleavage plane in lamellar keratoplasty, explains Sadeer B. Hannush, MD, an attending surgeon on the Cornea Service at Wills Eye Hospital and a professor of ophthalmology at Sidney Kimmel Medical College of Thomas Jefferson University in Philadelphia. “The pre-Descemet’s layer improves our understanding of DALK and makes it safer,” he says. “This layer is about 5 to 20 microns thick and is made of about five to 10 lamellae of collagen fibers. It’s continuous with the trabecular meshwork. Many cornea specialists believe the pre-Descemet’s layer to be acellular or paucicellular.”
The pre-Descemet’s layer is structurally strong. “If you only have Descemet’s membrane between the anterior chamber and the outside world, you need about 30 mmHg of pressure in the anterior chamber to pop through Descemet’s membrane,” Dr. Hannush says. “But in the presence of pre-Descemet’s layer, you need about 500 to 700 mmHg to pop through. This is a very unique property.” In the big bubble DALK technique, the pre-Descemet’s layer forms the posterior wall of the type-1 bubble and the anterior wall of the type-2 bubble (more below).
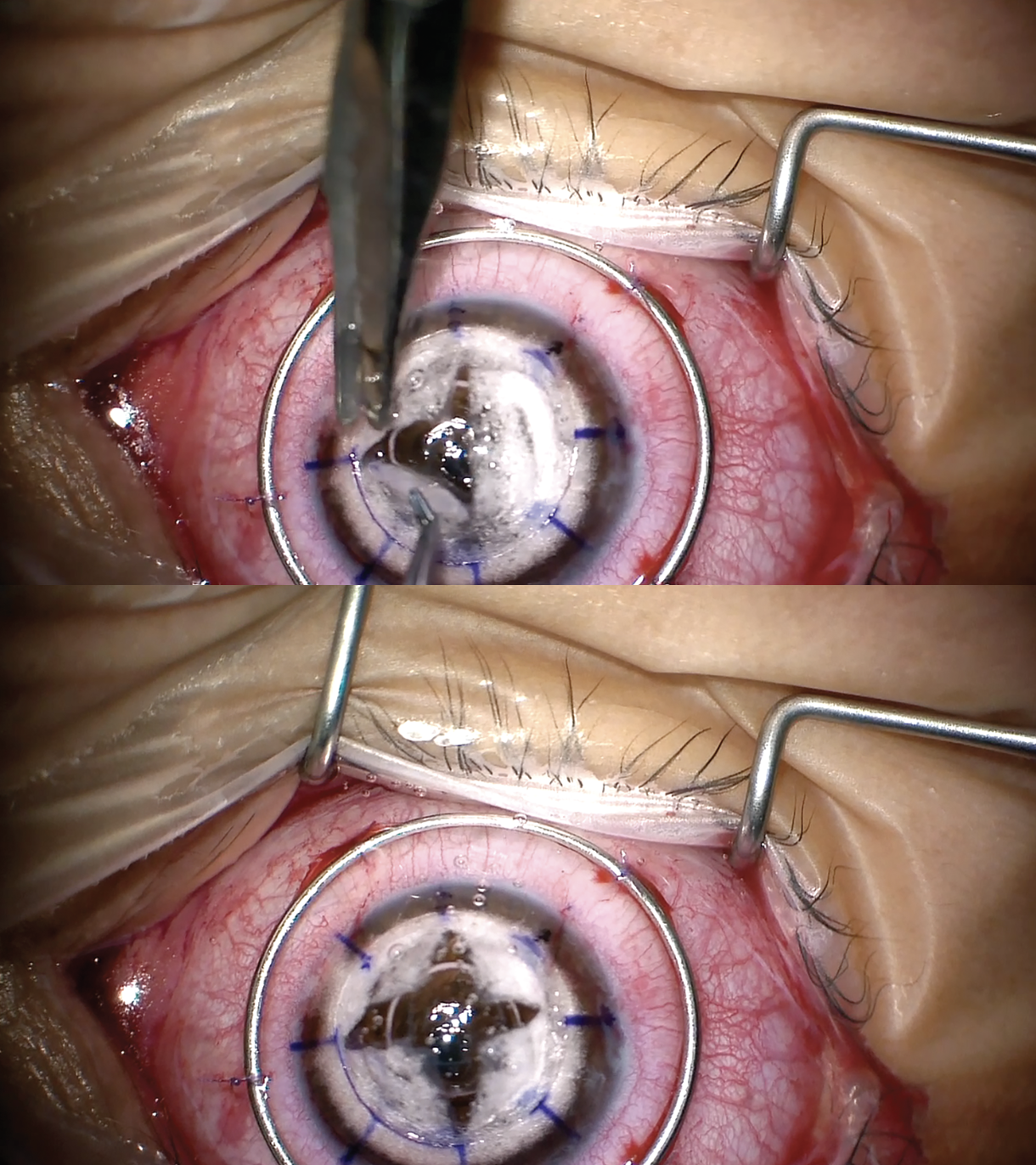 |
| Quadrant removal of the stroma is performed to expose Descemet’s membrane. |
The Big Bubble Technique
The big bubble technique uses air pressure to create a cleavage plane between the Descemet’s layer and the stroma. “Patients that have a relatively clean stroma without any scarring are better candidates for the big bubble technique,” Dr. Prakash says. “If patients have scarring or very thin corneas, we can do a manual deep LK rather than trying to do a big bubble. The big bubble technique requires blunt force to create a Descemet’s detachment, so if there’s a weak area of Descemet’s (e.g., previous hydrops) and we try to make a big bubble, that will go through and through from that area. If the patient has any scarring, that’s a potential area through which air can leak from the stroma into the anterior chamber, and then we can’t do a big bubble in that situation.”
Here are some pearls for performing successful big-bubble DALK:
• Invest in the best tools for the job. Using the right instruments such as blunt-tipped scissors, special blunt-tipped air cannulas and blunt lamellar dissectors “will pay dividends as it’ll decrease the risk of inadvertent perforations,” Dr. Jeng explains.
• Carefully evaluate preoperative corneal thickness. On preoperative tomography or OCT, examining peripheral corneal thickness is key. “Some of our keratoconus patients, which is one of the main indications for DALK, are very thin in the periphery and you really start cutting on your very first turn with the corneal trephine,” explains Kourtney H. Houser, MD, an assistant professor of ophthalmology and director of the cornea fellowship program at Duke University. “So, knowing the thickness in those very steep corneas beforehand is helpful for judging the appropriate depth of your trephination. There are some guarded and measured trephines that can make it a little easier to control depth.”
• Debulk the stroma first. “The big bubble technique works by creating an air cushion into the stroma, so the less stroma there is, the better,” says Dr. Prakash. “Especially for new DALK surgeons, I want them to debulk at least two-thirds of the cornea before they start attempting the big bubble, because then we can control the amount of air injected. It’s a very methodical approach.”
• When injecting the air bubble, go as deep into the stroma as possible. “Put the cannula as far posteriorly in the stroma as possible without going through and through to the anterior chamber,” Dr. Hannush says. “The opening of the cannula should point down toward the anterior chamber. Inject air. The pathway of least resistance for the air is to go posteriorly and split the stroma off of the pre-Descemet’s layer.”
“Correct depth is key,” Dr. Prakash adds. “Around 90 percent of the initial depth is what we want to go in when doing the big bubble technique. Also avoid injecting air too fast and hard. This can blow Descemet’s membrane.”
• Don’t use a needle to inject air. “Your chances of perforating are higher,” Dr. Hannush says.
• Inject air in a soft eye. “When you start injecting the air into the posterior stroma, you want the eye to be soft,” Dr. Hannush says. “It’s easier to get the cannula into the stroma in a firm eye, but once you get the cannula in, placement of a paracentesis to release some aqueous and soften the eye increases the likelihood of getting a big bubble.”
• Familiarize yourself with the appearances of a big bubble type 1 vs. type 2. When a big bubble type 1 appears, there’s no mistake about it, Dr. Hannush says. After a wave of intrastromal emphysema, the border of the bubble is smooth and expands from center to periphery. “You know that the bubble is anterior to pre-Descemet’s layer and you’re likely to be successful with your DALK after entering the bubble with a knife, a step known as the ‘brave slash.’ If it’s a big bubble type 2, it means the bubble is between pre-Descemet’s layer and Descemet’s membrane. There is little intrastromal emphysema, and the bubble expands from periphery to center. This is a fragile situation. Some surgeons recommend not entering the bubble with a knife, but rather performing a layer-by-layer deep dissection followed by transferring the donor graft on to complete the keratoplasty.
• Use a small bubble to check your technique. While intraoperative OCT can be used to see where the bubble is, many surgeons don’t have access to this instrument. “Another option is to place a small bubble on the periphery, which is called the double-bubble technique,” Dr. Prakash explains. Basically, once you’ve created a big bubble, you make a peripheral, almost vertical stab incision and put in a small bubble. That bubble should not move to the center, because the center of the anterior chamber is already being involved by the Descemet’s layer, which is pushed down by the big bubble. This gives you an idea of how good the bubble is.”
• Avoid over-pneumatizing the stroma. “Another problem newer surgeons may encounter is not getting the full cleavage in the first bubble,” Dr. Prakash says. “They may continue to put air in, which is okay up to a point. It’s important not to pneumatize the stroma, otherwise it becomes difficult to get a clear dissection. The technique for this is to use a targeted approach in areas which aren’t already pneumatized.”
• Begin stromal removal through viscoelastic. “If a big bubble is achieved and we’ve reached a point that we have to rupture the central stroma so we can get bare Descemet’s, I generally recommend that the surgeon put a blob of viscoelastic at the area where they’re making a nick on the stroma at the center,” Dr. Prakash says. “What happens is that once you make a nick in the central stroma, air releases and gushes out from the big bubble because it’s under tension. The Descemet’s isn’t supposed to be that stressed and the sudden release can actually break the Descemet’s when you’re trying to remove the stroma. Once you have a blob of viscoelastic and you go through that to make a nick, the [air] leak is slow, and the bubble doesn’t collapse as fast. The rate of hitting the bubble and creating a perforation is much less.”
Similarly, he says that newer surgeons may find it easier to use a needle instead of a blade, since the track is smaller, allowing for less air to leap back. “It’s always good to try a big bubble in a few animal eyes first to get an idea of how the air moves in your hand,” he adds.
• Visualize first, then cut when dissecting out the cleavage plane. “Once we’ve reached the bare Descemet’s and are making four quadrants, it’s important to make sure the nicks on the stroma are in to out,” Dr. Prakash says. “Avoid hitting the Descemet’s when you’re trying to do quadrant removal of the stroma. Most perforations happen in the periphery, which happens because surgeons lose the guidance of how deep they have to go when they try to cut on the peripheral stroma. I always suggest visualizing first, then cutting—a basic tenet of lamellar keratoplasty. You should always lift up the cornea, which has to be dissected out to see where your scissors are going. Then, cut the periphery. This ensures you won’t inadvertently catch the Descemet’s membrane when you’re trying to cut at the periphery.”
• If a microperforation occurs, don’t inject viscoelastic. “The tendency for surgeons when they perforate is to immediately grab viscoelastic to try to reinflate the eye,” Dr. Jeng says. “This is a major mistake that can be avoided by injecting air instead. Air will tamponade the perforation, whereas viscoelastic will expand the perforation and make it bigger. Microperforations can be salvaged by carefully dissecting in other areas first and then leaving a little bit of stromal tissue over the perforation site if off axis.”
• Ensure the posterior stroma is completely separated from pre-Descemet’s layer. “When removing the posterior stroma, ensure the complete separation of the posterior stroma from Descemet’s membrane all the way to the periphery, so that when you start cutting the posterior stroma to remove it, you don’t nick pre-Descemet’s layer with your scissors,” Dr. Hannush says.
• Pay attention to depth when suturing. “When most surgeons do PK, they [pass the needle at approximately] 90-percent depth in the host and the donor,” Dr. Prakash says. “[For DALK], one of my good friends and colleagues, Mayank A. Nanavaty, recommends suturing at 50-percent depth in the donor and 90-percent depth in the host to improve apposition of the donor with Descemet’s membrane, which I have started doing after reading his work.2 I agree that this makes operation much easier, which sounds counterintuitive for most. The reason is that it creates a tug and pulls the donor back into the host, which is what we want in these situations. That’s what I’ve been teaching my fellows. So, rather than doing 90 percent on both sides, try to be more superficial on the donor and deeper on the host. This also reduces the risk of hitting the bare Descemet’s membrane when you’re putting in your first few sutures.”
• Don’t give up. There’s a learning curve with the big bubble technique, but practice pays off. “Most of us like to do the big bubble technique where we inject air and try to get a full cleavage of the plane between Descemet’s and the stroma, but if you don’t achieve a big bubble you can still be successful at DALK, you just have to be patient in a manual dissection,” says Dr. Houser.
DALK vs. Manual Dissection
Surgeons may turn to manual dissection when a big bubble can’t be achieved, or they may begin with manual dissection from the outset. “It’s more laborious to remove the cornea layer by layer until we have a nice reflection back to the Descemet’s,” Dr. Prakash points out. “In fact, it’s very difficult to get a bare Descemet’s membrane in this situation and leaving about 25 to 30 or 50 microns may be better than trying to go deeper and potentially perforating.”
Clarity is a key difference. “Bare pre-Descemet’s layer is pristine, and its optical quality is excellent,” Dr. Hannush says. “Manual dissection can’t achieve as optically clear a situation as by baring pre-Descemet’s layer. I get a big bubble about 80 percent of the time, and the other 20 percent of the time I perform manual dissection, always making an effort to retain the host Descemet’s membrane and endothelium.”
Viscobubble DALK
If air bubble formation fails, which is common even among experienced surgeons, manual dissection is often the next fallback, but recently another approach has shown promise. “Viscoelastic can also be used as a dissection force instead of using air,” Dr. Prakash notes. “Surgeons who use this technique feel that it’s a bit safer to use viscoelastic force [than air].”
A study of 140 keratoconus eyes on the outcomes of bubble formation with OVD after failed air bubble dissection for DALK reported that this approach increased the success rate of bubble formation from 75.71 percent to 95.71 percent.3 They noted, however, that when bubble formation fails, the infiltration of OVD into the residual stroma complicates manual dissection, also resulting in interface haze and poor visual outcomes. No significant differences were noted between air and OVD bubble at later examinations.
Femto-assisted DALK
Femtosecond lasers such as the IntraLase and the Z8 can be employed to make both the trephination and the posterior lamellar pass. “The posterior lamellar pass can be done predictably, so you can program the laser to do it at 100 to 120 microns from the endothelium,” Dr. Hannush says. “What is the tolerance? Can there be error? Can you perforate? Sure. However, some surgeons feel that this can be done predictably and safely allowing a better chance for the creation of a big bubble.”
Alternatively, the laser can also be used for trephination only followed by creation of the big bubble. “Once the laser makes a pass, you’re committed to surgery at that level,” Dr. Hannush points out. “You can still create a big bubble by injecting into the 100-micron-thick residual cornea. This is an alternative to the conventional approach for creation of the big bubble, but most surgeons won’t use the femtosecond laser to make a horizontal pass.”
Dr. Prakash says that femto-assisted DALK can be helpful for patients who have herpes scars or keratoconus with hydrops, where you don’t want to destabilize the cornea by doing a big bubble. “We can make a femtosecond-assisted lamellar cut or make a partial trephination,” he says. “The benefit of the femtosecond is that it’s much more precise and it doesn’t depend on how hard or tight you use the vacuum trephine. So, you can debulk the anterior two-thirds of the cornea with this technique, and the remainder can be done by manual dissection. That’s one technique for deep LK.”
When to Convert to PK
“If [the big bubble technique is] done successfully, generally the DALK is able to be completed without any issues,” says Dr. Jeng. “Surgeons may run into trouble when they can’t get a big bubble and end up needing to do a manual dissection. As you try to dissect deeper and deeper into the stroma, there’s a greater chance of getting too close and perforating [Descemet’s layer].
“Conversion is only done when there’s an intraoperative rupture of Descemet’s membrane,” he continues. “If it’s a microperforation, then this can usually be salvaged and the DALK can be completed. If it’s a macroperforation, sometimes it’s not possible to continue because the hole is too big, and that’s when most surgeons convert to a full-thickness transplant.”
Surgeons have different thresholds for converting to full-thickness transplants, but in the United States, the threshold is relatively low compared with other countries. “[In the United States] we perform about 50,000 corneal transplants but have about double the amount of donor tissue,” Dr. Hannush explains. “We export the rest. Since we have access to excellent corneas with healthy endothelium, a surgeon who doesn’t have much experience with DALK or perforates early into the anterior chamber can easily convert to a full-thickness transplant knowing that the patient will do well with a full-thickness donor cornea. However, in many parts of the world, donor tissue with healthy endothelium is less readily available, so being able to perform a successful DALK is possible with tissue that may not be good for full-thickness transplantation.
“Some colleagues will order two corneas, one with borderline or poor endothelium, which they’ll use if they’re successful in creating a big bubble, and one with an excellent endothelium, which they’ll use if they have to convert to a full thickness transplant,” he continues. “This is a luxury that U.S. corneal surgeons have. The surgeon may then send back the unused full thickness donor tissue within a few hours, so it can be placed elsewhere.”
Dr. Hannush says his threshold for converting is “when I have more than 3 to 4 clock hours of peripheral laceration of Descemet’s membrane. If the perforation is central and small, there’s no need to convert. A layer-by-layer dissection may be carried out, since it’s difficult to create a big bubble in the presence of a full-thickness opening into the anterior chamber.”
Dr. Houser says, “If I’ve penetrated the host endothelium and Descemet’s on my trephination cut, then I’ll usually convert to PK at that time because it can be very challenging to try to manually dissect off the stroma if you have a large penetration into the eye on your initial trephination. If there’s a small break in Descemet’s during the dissection after the trephination, and it’s peripheral, I’ll usually not convert and instead try to continue with a manual dissection to complete the DALK. I’ll try to avoid that area during the manual dissection and then dissect around that area last, trying not to propagate the tear. Then I’ll put a bubble into the anterior chamber at the end of the case. But, if there’s a very large, central perforation, or if I’ve penetrated on my original trephination, then I usually convert to PK.”
How do surgeons talk to patients about the potential for conversion to a full thickness transplant? “I usually tell patients that if it’s possible, I like to do a DALK because it may have a lower rejection rate, we can remove the sutures sooner and use less steroids [than a PK],” Dr. Houser explains. “There are multiple reasons why, if we can do a DALK, it may be favorable for them. But I tell patients that sometimes we’re not able to do a DALK—their tissue may be too thin; we may penetrate into the eye and have to do a penetrating keratoplasty. I usually tell patients that we have everything ready to do both procedures. I usually order just penetrating keratoplasty tissue and then remove the endothelium should I be able to do a DALK. Some surgeons, and I’ve done this in the past as well, will order DALK tissue and PK tissue and send back the one they don’t use. I’ll assure patients that I have everything to do both procedures and whichever one seems to work best for their eyes is what we’ll end up doing.”
Postoperative Complications
A double anterior chamber is the most common postoperative DALK complication. “A double anterior chamber is when there’s either saline, aqueous fluid or viscoelastic material in the space between the donor and pre-Descemet’s layer,” says Dr. Hannush. “In that scenario an air bubble is placed in the anterior chamber and the fluid in the interface is burped out through the graft-host junction. This may be done at the slit lamp or in the operating room. Like with DMEK or DSAEK, the patient is then positioned supine for a short period of time, allowing Descemet’s membrane and the pre-Descemet layer to be approximated to the donor graft.”
Donor and host mismatch can also result in a double anterior chamber. “If you oversize the donor button, there can be a bit of a mismatch and you can have a hard time getting the host tissue to stick to the donor,” Dr. Houser says. “Usually, your re-bubble works in those cases, but sometimes you have to incise the graft-host junction area and re-suture it. Or, if you have some sutures that are much tighter than others, you may need to replace those to make them a little more uniform.”
The incidence of stromal rejection is low, but it can occur with DALK. “Because we haven’t replaced the endothelium, the risk of endothelial rejection is decreased, but stromal rejection does happen in DALK,” Dr. Prakash says. “If it’s not reversed, it can lead to scarring. In that case, you could peel off the old graft and place a new one.”
“In the event of stromal rejection, blood vessels may grow into the interface which can be problematic,” Dr. Hannush says. “One way to manage that is to ensure the patient comes back for their scheduled visits. If you see edema of the graft or early vessels growing into the interface, frequent topical steroids may be initiated or an injectable steroid placed in the sub-Tenon’s or subconjunctival space. If DALK was done for a herpes simplex scar, then make sure the patient is also on oral antivirals.”
Interface haze can also occur, but experts say it’s usually a small contributor to visual loss. “There are several ways to decrease haze,” he continues. “You can use steroids and vitamin C like we do after PRK, and ensure the patient wears dark sunglasses if the surgery is done in summer to minimize exposure to UV light. There also may be a role for the antihypertensive losartan.
“Please consider DALK if the host corneal endothelium is healthy,” Dr. Hannush says. “Consider it for corneal ectasias like keratoconus. Consider it for stromal scars and for most corneal stromal dystrophies. Don’t abandon the option of DALK for penetrating keratoplasty without at least trying to do DALK. And if you fail, you can also always convert to a full-thickness transplant.”
Dr. Prakash, Dr. Jeng, Dr. Hannush and Dr. Houser have no related financial disclosures.
1. 2023 Eye Banking Statistical Report. Eye Bank Association of America, 2024.
2. Nanavaty MA, Singh Vijjan K, Yvon C. Deep anterior lamellar keratoplasty: A surgeon’s guide. J Curr Ophthalmol 2018;30:4:297-310.
3. Scorcia V, De Luca V, Lucisano A, et al. Results of viscobubble deep anterior lamellar keratoplasty after failure of pneumatic dissection. Br J Ophthalmol 2018;102:1288-1291.