Retina: Imaging, Endpoints and Anti-VEGF
In recent years there’s been a growing focus at ARVO on the back of the eye, especially on those conditions with the fewest therapeutic options and greatest impact on visual health. Age-related macular degeneration, especially the non-exudative, or dry, form of the disease, is increasingly a focus of both basic and clinical research.1,2 As such, understanding the course and risk factors of AMD, as well as evaluating new imaging techniques and clinical endpoints, were hot topics at this year’s meeting.
AMD is a slowly progressive disease characterized in its early stage by extracellular drusen deposits and pigmentary changes in the macula. Drusen features, including size, are important risk factors for the progression of more-advanced forms of AMD. However, the exact role of drusen in the pathogenesis of AMD is not fully understood. One report (Baratsits, et al;ARVO E-abstract 1579) demonstrated that eyes from early AMD subjects showed both a dynamic progression and a regression of drusen volume, which, surprisingly, occurred simultaneously at different locations within the same retina. Another group looked for molecular changes in drusen using fluorescence lifetime imaging ophthalmology (FLIO) (Sauer, et al;ARVO E-abstract 3399). This technique distinguishes soft and hard drusen based upon autofluorescence; using this method it was possible to differentiate drusen types, distinguishing those in patients with AMD from those with healthy retinas; thus, giving FLIO the potential to provide information on individual risks for the progression to later stages of dry AMD.
Progression to more advanced stages of AMD is characterized by geographic atrophy and choroidal neovascularization. In patients with intermediate AMD, the risk and speed of progression to GA or CNV is highly variable. A study by Erfurth, et al (ARVO E-abstract 3398) showcased a fully automated machine-learning method that was developed to predict progression to AMD based on retinal imaging and genetics. Fellow eyes with intermediate AMD from subjects enrolled in the HARBOR trial were used to demonstrate that an automated analysis of OCT biomarkers allowed for a personalized prediction of AMD progression. Interestingly, genetic characterization didn’t add additional accuracy to the prediction of AMD conversion.
As another means of predicting progression of GA or CNV, Chakravarthy, et al (ARVO E-abstract 2979) presented results from a large U.K. cohort study (83,425 persons from 10 U.K. eye clinics) demonstrating that progression to GA or CNV was frequently observed in eyes with early to intermediate AMD, and was more common in patients with advanced disease in the contralateral eye.
A major limitation in the development of novel therapeutic options for AMD has been a lack of sensitive endpoints to evaluate their therapeutic efficacy. Although using GA growth rate is currently an accepted endpoint in clinical trials, therapeutic intervention at this advanced stage may have little impact on preserving a patient’s sight. A study by Bagheri, et al (ARVO E-abstract 3400), carried out a retrospective review of blue autofluorescence fundus images, optical coherence tomography and Snellen visual acuity in patients with GA due to AMD (18 subjects) in order to understand the relationship between total GA, foveal GA and VA in AMD. Results suggest that the percentage of GA within the fovea may be more sensitive in predicting the degree of visual acuity impairment than the total GA
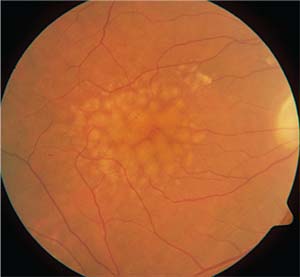 |
| One of the highlights from this year’s ARVO meeting was a report that found that taking a closer look at drusen characteristics may provide an improved diagnostic protocol for the non-exudative form of age-related macular degeneration. |
An additional study by Cocce, et al (ARVO E-abstract 3765), further elaborated on the lack of reliable functional endpoints for proof of concept clinical trials in AMD through the evaluation of visual function metrics. This study demonstrated that low-luminance visual acuity, mesopic microperimetry with eye tracking, cone contrast test, or dark adaptation may be used as potential functional measures in clinical trials involving patients with early and intermediate AMD. The theme of developing sensitive endpoints for the clinical study of retinal degeneration was demonstrated in a number of presentations. These studies evaluated the use of a variety of visual tests including critical flicker fusion (Slocum, et al;ARVO E-abstract 2347; Lane, et al;ARVO E-abstract 4714), photostress and photobleach (Rodriguez, et al;ARVO E-abstract 4715), or critical flicker fusion combined with photobleach (Sundstrom, et al;ARVO E-abstract 4713) to examine the potential of these visual tests to serve as sensitive endpoints for AMD.
Although there are no currently approved treatments for dry AMD, there are several commonly prescribed treatments for the neovascular, or wet, form. These include: ranibizumab (Lucentis) and aflibercept (Eylea). Both therapies are intravitreal injections that antagonize the angiogenic factor, VEGF. While data from clinical trials have demonstrated the effectiveness of these therapies for the treatment of wet AMD, real-world data on the long-term effects of treatment are lacking.
Several studies at ARVO evaluated the effectiveness of aflibercept after long-term treatment. These studies demonstrated the long-term effectiveness of aflibercept in the treatment of wet AMD after three years (Babalola, et al;ARVO E-abstract 423); however, visual acuity was lower for subjects who didn’t complete three years of treatment (Vig, et al;ARVO E-abstract 411) and longer treatment was needed for subjects with persistent disease activity (Eleftheriadou, et al;ARVO E-abstract 874). A study by Queguiner, et al (ARVO E-abstract 414), demonstrated that ranibizumab injections were effective for up to five years of treatment, raising the question of whether ranibizumab is a more effective treatment than aflibercept for wet AMD. Interestingly, Gillies, et al (ARVO E-abstract 896), did a 12-month, head-to-head comparison that looked at visual acuity changes in 394 eyes with wet AMD; there were no significant differences in acuity between patients treated with ranibizumab compared to aflibercept for 12 months, suggesting both drugs are effective for the treatment of wet AMD in real-world clinical practice.
Since current therapies for wet AMD require repeated intravitreal injections of anti-VEGF, numerous presentations at ARVO focused on new delivery mechanisms, many of which focused on extended-release formulations that would not only reduce patient burden but potentially provide improved clinical outcomes. Owens, et al (ARVO E-abstract 409), from Envisia Therapeutics demonstrated a proof-of-concept, multi-month release of aflibercept from a biodegradable hydrogel matrix in vitro that was further supported by three-month-release data in non-human primates. A bio-absorbable hydrogel depot delivery system was also described in a presentation by researchers from Ocular Therapeutix, but in this case the device was used to deliver a tyrosine kinase inhibitor (Jarett, et al;ARVO E-abstract 1956). In rabbits, the device delivered and maintained high levels of tyrosine kinase inhibitors in the vitreous humor, retina and choroid for six months. Parallel studies of this same delivery system demonstrated that it could provide six months of therapeutic levels of drug treatment for wet AMD using a VEGF-induced retinal leakage model (Elhayek, et al;ARVO E-abstract 1968).
There was also great interest in studies and treatments for inherited retinal disease. While this group of disorders doesn’t have the prolonged natural history that makes AMD so difficult, developing treatments and establishing valuable clinical endpoints remain a challenge. One exciting gene therapy approach may soon bring a new treatment option for retinitis pigmentosa patients to clinical trials. ProQR Therapeutics presented new data demonstrating that their antisense oligonucleotide product, QRX411, restores wild-type USH2A RNA in RP patient-derived optic cups (van Diepen, et al;ARVO E-abstract 1203). As mutations in the USH2A gene are known to be a cause of RP, the ability to correct mutations in vivo might make it possible to stop disease progression and vision loss in these patients. QRX411 was also effective in vivo in a USH2A mutant zebrafish model. QRX411 has obtained orphan designation from the European Medicines Agency, and we are excited to watch this new therapy make its way to the clinic.
Of course the ultimate retinal therapy, stem cell replacement of damaged retinal tissues, is still years away. This field was on the front burner in two recent, back-to-back papers that described two different approaches to stem cell replacement in patients with AMD.3,4 In one study, autologous fibroblasts were differentiated into a sheet of retinal pigmented epithelial cells; the transplanted cellular sheet was healthy one year after transplant, though the patient didn’t achieve any improvement in vision.3 The second paper described the adverse effects of injection of adipose-derived stem cells in three patients, all of whom developed ocular hypertension, hemorrhagic retinopathy and severe vision loss.4 In this setting the mini-symposium, Novel Therapies and Imaging Techniques for Retinal Disorders (Sohn, E;ARVO E-abstract 2467; and others) provided a comprehensive background on the status of this groundbreaking therapeutic path.
Even the most
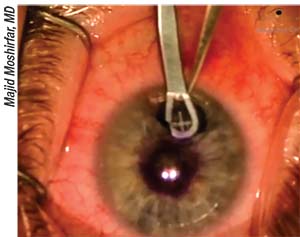 |
| Researchers have found that the AcuFocus Kamra inlay may benefit from incremental design changes. |
Cyclosporin A is now commercially available as an oil-based surfactant for dry eye and other inflammatory disorders, but its use is limited by low aqueous solubility. Surfactants that are used to improve solubility can irritate the surface of the eye, causing burning, itching and irritation of the conjunctiva. One presentation (Johannsdottir, et al;ARVO E-abstract 4442) reported on a preclinical study designed to develop a surfactant-free drop by delivering cyclosporin A via cyclodextrin nanoparticles. The eye-drop formulation with cyclodextrin nanoparticles exhibited no ocular irritation in rabbits after three months of treatment. A similar clinical study compared the postoperative use of 1.5% dexamethasone nanoparticle-containing eye drops (Oculis; Reykjavik, Iceland) with mitomycin-C and Maxidex as follow-up after trabeculectomy (Stefansson, et al;ARVO E-abstract 4947). This was a randomized, double-masked clinical trial with 25 patients undergoing trabeculectomy for poorly controlled primary open angle glaucoma. (The study team was composed of both consultants and employees of Oculis.) The researchers say that DexNP eye drops were an effective post-trabeculectomy treatment and may serve as an alternative to current treatment used in conjunction with glaucoma surgery and other conditions where anti-inflammatories are indicated.
The Front of the Eye
Presbyopia isn’t as debilitating as retinitis pigmentosa or AMD, but the decrease in near vision that accompanies it does result in some level of disability that can impact one’s quality of life. A presentation by Aston University’s James Wolffsohn, PhD, from Birmingham, U.K., (ARVO E-abstract 2996) was part of a larger mini-symposium on the aging eye that discussed the optical and visual trade-offs associated with lenses for presbyopia correction. These included well-known non-surgical options such as contact and spectacle lenses, as well as more novel surgical approaches like diffractive/refractive aspheric optics, trifocal lenses, and “accommodating” intraocular lenses. Gutierrez-Contreras, et al (ARVO E-abstract 1246), demonstrated the promise of accommodating intraocular lenses for restoring accommodation levels similar to the crystalline lens’s at a presentation session focused on intraocular lenses and presbyopia correction.
One of the latest solutions for presbyopia is the Kamra inlay. This inlay works by limiting the size of the effective pupil to 1.6 mm, thereby taking advantage of the paraxial behavior of light. Work presented by de Gracia and Hartwig (ARVO E-abstract 327) sought to optimize the optical design of the Kamra inlay. This study used computer-simulated variations of the inlay design, then tested their fit and performance in silico for 1,299 subjects. They were able to show that slight incremental design changes such as a reduction in the number of holes could improve the optical properties of the inlay.
Due to the limitations inherent in the surgical correction of presbyopia, the use of pharmaceutical therapy has been explored but, thus far, continues to be limited. Encore Vision/Novartis had a strong presence during the presbyopia poster session with the presentation of their novel eye drop for presbyopia correction: EV06. In a Phase I/II study, treatment of presbyopes with EV06 resulted in a statistically and clinically significant improvement in distance-corrected near visual acuity when compared to placebo (Korenfeld, et al;ARVO E-abstract 331). A seven-month follow-up of this Phase I/II study demonstrated that the gains in distance-corrected near visual acuity, attributed to dosing with EV06 for 90 days, persisted for at least an additional 210 days after final exposure to the drug (Stein, et al;ARVO E-abstract 330).
Dry Eye
Efforts to characterize dry eye at the gene and protein level are being met with increased interest as clinicians and basic scientists alike look for new ways to diagnose and describe the disease. For those developing new therapies for dry eye, the discovery of differential gene/protein expression between healthy subjects and those with the disease might mean new therapeutic targets. One group used a mouse model of severe dry eye to explore changes in the expression of inflammation-associated genes (Daull, et al;ARVO E-abstract 797). Inflammation is a key driver of some of the signs and symptoms of dry eye. Results from the study showed either an up- or downregulation of 34 genes, which may translate to therapeutic targets or diagnostic markers after future studies assess the role that these genes play in human disease.
In a study investigating differences in protein expression between healthy subjects and those with dry eye, differentially expressed proteins were discovered in tear samples from the two groups (Perumal, et al;ARVO E-Abstract 796). Proteins associated with increased inflammation were significantly increased in the dry-eye group, while some proteins necessary for tear function and ocular health, such as lysozyme and proline-rich protein 4, were significantly decreased. In total, 76 differentially expressed proteins were discovered in three main categories: inflammation; apoptosis; and metabolism. The results of these studies may lead to clinically useful biomarkers for patient diagnosis and clinical trial endpoints.
Results from tests of a new lubricant eye drop for patients with lipid-deficient tears were shown this year (Hom, et al;ARVO E-Abstract 2671). The novel eye drop contains lubricant polymers along with omega-3 fatty acids and trehalose. When compared to a marketed eye drop, the investigational drop proved non-inferior for symptomatic dry-eye relief. Further, subjects using the investigational drop saw significant improvement in corneal and conjunctival staining when compared to the control drops at all follow-up visits.
One particularly exciting topic in the clinical dry-eye community at ARVO this year was Allergan’s TrueTear Intranasal Tear Neurostimulator. The TrueTear is a small, handheld device with two slim prongs that deliver a small electrical current to sensory neurons in the nasal cavity. This stimulation induces the nasolacrimal reflex, resulting in tear formation. In a trial of 25 dry-eye subjects, the TrueTear was shown to significantly increase tear production when measured by Schirmer’s test and tear meniscus height measured by optical coherence tomography imaging (Orrick, et al;ARVO E-Abstract 2692). In the same study, it was shown that TrueTear use also significantly increased tear film lipid layer thickness, a finding that may have implications in tear-film stability for patients with evaporative dry eye (Watson, et al;ARVO E-Abstract 4387). A separate study analyzed meibomian gland morphology before and after TrueTear stimulation in subjects with dry eye (Pondelis, et al;ARVO E-Abstract 2235). The group used OCT imaging to measure the size of the glands and found that overall gland area and perimeter was significantly smaller after stimulation. The authors suggest that this is due to expression of meibum from the glands, a finding that corroborates the results from the Watson study described above. With the recent FDA market authorization, we’re sure to see much more of the TrueTear in the clinic very soon.
We also saw positive results presented from Regentree’s ARISE-I Phase IIa study evaluating RGN-259 (Thymosin β-4) ophthalmic solution for the treatment of dry-eye syndrome (Ousler, et al;ARVO E-Abstract 2668). This five-week, multicenter study evaluating 317 subjects in three arms (placebo and 0.05% and 0.10% RGN-259) showed positive results for RGN-259 in measures of ocular discomfort and ocular surface staining compared to placebo. The 0.05% and 0.10% solutions reduced ocular discomfort on day 28 both before and, in patients more symptomatic at baseline, after exposure to a controlled adverse environment. Additionally, subjects in the 0.05%- and 0.10%-RGN-259 groups with worse tear-film breakup time at baseline saw improved corneal staining scores by the end of the study. We’re looking forward to more positive results from future studies.
ARVO 2017 also hosted a special-interest group in which conclusions and recommendations from the TFOS Dry Eye Workshop II were presented (Sullivan, et al). This update of the 2007 DEWS report includes refinements in endpoints and greater emphasis on neuropathic aspects of dry eye. Details should be available with publication of the full report later this summer. Allergy and dry eye were also highlighted in a mini-symposium focused on the relation of these conditions to ocular surface health. It’s well known that the allergic response begins with the engagement of allergen by IgE receptors on the surface of mast cells, resulting in an inflammatory cascade primarily composed of histamine and other local mediators. Symposium presenters described the latest work on shared immune mechanisms seen in both allergic disease and dry eye. In one of these presentations, Andrea Leonardi, MD, of Italy’s Padua University, discussed how disruption of cell-cell junctions on the ocular surface is pivotal to the immune response, and how analysis of patterns of tissue RNA from the conjunctival surface has provided clues to the underlying etiology of the severe allergic disorder vernal keratoconjunctivitis (ARVO E-Abstract 1599). These results demonstrated a distinct conjunctival transcriptome involved in immune signaling, and thus they’ve provided new potential drug targets for the treatment of severe ocular allergy.
In this report, we’ve only covered a tiny fraction of the posters, talks, symposia and special-interest groups that were on display at this year’s ARVO meeting. Looking ahead, although Baltimore was a great venue, it will be hard-pressed to compete with next year’s meeting in Hawaii. See you there. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School. Dr. Hollander is chief medical officer at the ophthalmic consulting firm Ora in Andover, Mass., and an assistant clinical professor of ophthalmology at the Jules Stein Eye Institute at the University of California, Los Angeles.
1. Sacconi R, Corbelli E, Querques L, Bandello F, Querques G. A review of current and future management of geographic atrophy. Ophthalmol Ther 2017;6:1:69-77
2. Villegas VM, Aranguren LA, Kovach JL, Schwartz SG, Flynn HW Jr. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin Drug Deliv 2017;14:2:273-282.
3. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;16:376:11:1038-1046.
4. Kuriyan AE, Albini TA, Townsend JH et al. Vision loss after intravitreal injection of autologous stem cells for AMD. N Engl J Med 2017;16:376:11:1047-1053.



