Mark B. Abelson, MD, CM, FRCSC, Aron Shapiro, CierraMaffei,
You've successfully evaluated the patient's medical history, assessed ocular signs and symptoms with the many tools available to you and performed the necessary diagnostic tests to clinch the diagnosis. Then, after meticulously weighing the best evidence-based treatment for your diagnosis, you've written the prescription. While you may think the toughest tasks are behind you, the biggest challenge to proper treatment has actually just begun: patient compliance.
By entrusting the patient with the prescription medication, you furnish him with a tremendous amount of control over his treatment, and ultimately the medical outcome. Once he heads home, you have very little (if any) control over when and how he uses his medication.
In this month's column, we'll discuss some strategies to ensure maximal compliance from your patients before they walk out of the office with their prescription.
Patient Education
Patient education is one of the most important tools the clinician can use to help ensure proper compliance. Medication-related adherence rates are estimated to hover around 50 percent, indicating that poor adherence is a widespread problem.1 Though increasing adherence in your practice may require a few extra minutes with patients, it has the potential to prevent future ocular problems. Also, in the case of a younger patient, both the patient and his parents should be educated.
First, carefully explain the ophthalmic diagnosis or situation and the benefits of treatment. If the patient comes in with dry eye, for example, explaining the importance of ocular surface hydration, the role of the blink in replenishing the tear film and the value of treating the condition regardless of whether or not symptoms are present (i.e., recommending use of an ocular lubricant four times daily) adds context for the patient.
Introducing the hazards of non-compliance is also vital to ensure patient comprehension. When prescribing an anti-infective agent for a case of bacterial conjunctivitis, for example, explaining the risks of spreading and sequelae, as well as the risks of increasing bacterial resistance with non-adherent dosing regimens, can help the patient understand what's at stake. Urging patients to adhere to an intensive dosing regimen for cases of microbial keratitis while introducing the dire, sight-threatening risk of ulceration is another example of an instance where education may be able to increase compliance.
Mention of these risks requires a careful balance, of course. You don't want to scare the patient. Instead, you need to be sincere enough to encourage comprehension of the gravity of the situation.
Urging patients to continue treatment de--spite any perceived symptomatic relief is also important.
Querying patients about potential concerns regarding treatment gives you another opportunity to identify and clarify sources of non-compliance. If the patient is concerned about cost or side effects, opening the door for discussion of these concerns can pre-empt any potential setbacks in his compliance.
This is also a great time to mention common side effects. If a drop is known to cause burning upon instillation in a substantial proportion of patients, it might be worthwhile to bring this up as a somewhat expected effect. A simple warning that this and any other common side effects are possible, but that the effect should be transient, may be enough to assuage a patient's anxiety. Also, with glaucoma patients, redness can develop with use of prostaglandin analogs, so be sure to inform the patients that the redness may actually be a sign that the drug is working. This is also a good time to educate the patient about the warning signs of serious side effects, such as adverse drug reactions, that warrant stoppage of the drug and a return to your office.
Drop Administration
Once you've convinced the patient of the importance of taking the medication, the next hurdle is to get him to actually take it.
Though instilling an eyedrop has some advantages over taking a pill, instilling the drop is far more complex. A substantial proportion of people taking eyedrops exhibit difficulty following proper procedures, and one study found that an alarming 69 percent of patients experiencing drop administration problems wouldn't report these problems to their doctors.2 Here, once again, we find an opportunity for pre-emptive action and education.
Inform the patient that proper instillation of eyedrops requires:washing of the hands;
after opening the container, checking the dropper tip carefully for any chips, cracks, plastic shards, etc; tilting the head and pulling down the lower lid to form a pouch for the drop once it's instilled; gently squeezing the bottle for controlled instillation (i.e., expelling one drop at a time) into the lower lid pouch and avoiding contact of the dropper tip with the ocular surface; closing the eyes after instillation; replacing the cap on the multidose container or disposing of the single-dose vial.
Also, avoiding contamination of the dropper tip via contact with the tear film is especially important so as to avoid biofilm formation. Demonstrating this procedure on yourself or patients using a sample of artificial tears in the office and then asking them to practice in front of you can reveal errors in application and allow you to correct them before the patient makes the same mistakes at home.
Asking patients to practice instilling drops in front of you may be especially helpful in improving administration. In one survey, over half of the patients taking eyedrops reported trouble administering the drops, with the most frequent trouble areas being the ability to direct the bottle and squeeze it appropriately.2 By watching and critiquing patient practice, the clinician has the opportunity to improve administration techniques and observe potential errors firsthand.
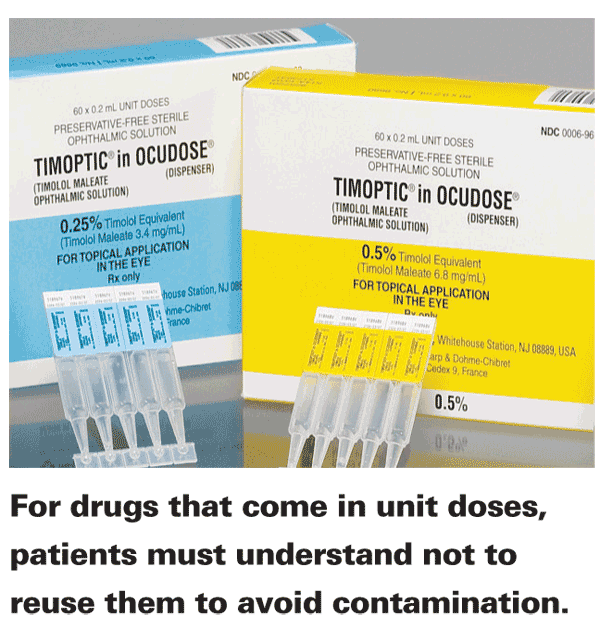
Emphasis should also be placed on using unit-dose products only one time. A unit-dose vial offers the convenience of on-the-go availability and a preservative-free option for patients who require or prefer it, but it tends to be more expensive than a multidose alternative. For this reason, patients using unit-dose products who notice leftover liquid after the first use may attempt to recap the product for use at a later time. While economical, this process may introduce added risk to the eye. Because these vials are developed and labeled for single use, they are not held to the same testing standards as multidose containers. There aren't any preservatives in the product, so microbial contamination becomes a valid concern as soon as the vial is opened to environmental factors and patient contact. In one study of 242 eyedrop bottles containing preservative-free artificial tears, for example, 2 percent demonstrated bacterial contamination 10 hours after use.3 While this percentage of contamination may seem small at first glance, it represents an avoidable risk, and one that may increase with extended periods of time after opening.
In unpreserved solutions, a formula's ingredients may also be of concern, especially when unit-dose products are used repeatedly over time. Carboxymethylcellulose, for example, is an ingredient in various ophthalmic solutions, but is also used as a medium for bacterial growth in various fields of microbial research.4 While sterility testing standards maintain that eyedrop solutions are free of bacterial contamination prior to use, any environmental exposure may increase the risk of microbial contamination of the solution, especially in subsequent uses.
The safest use of unit-dose products is detailed directly on their packaging with statements complying with federal regulation (e.g., "do not reuse," "once opened, discard")5 and in the prescribing information, such as, "Patients should be instructed that the solution from one individual single-use vial is to be used immediately after opening for administration to the affected eye. The remaining contents should be discarded immediately after administration."6 Pointing this out to patients and emphasizing its importance will, one hopes, drive the point home. Although researchers have examined the effects of refrigerating unit dose vials between uses in order to extend shelf life, the results are largely inconclusive.7
Other Compliance Tips
Other tips for increasing patient compliance include strategic planning with dosing regimens, improving accessibility to the drops and dosing duration considerations.1 If patients are forgetting (or anticipating trouble remembering) to take their drops on time, the clinician may find it helpful to suggest timing the drops around daily activities. These can include using the treatment at breakfast, lunch, dinner and bedtime, or around other regularly occurring activities, such as brushing teeth or taking other medications.
Catering to the patient's individual schedule may decrease the likelihood of skipped or late doses. Along the same lines, if the medication is packed in unit-dose packaging, clinicians can suggest that the patient leave a few of the doses at her workplace, in the glovebox of her car or anywhere else she frequents (taking temperature restrictions into consideration, of course).
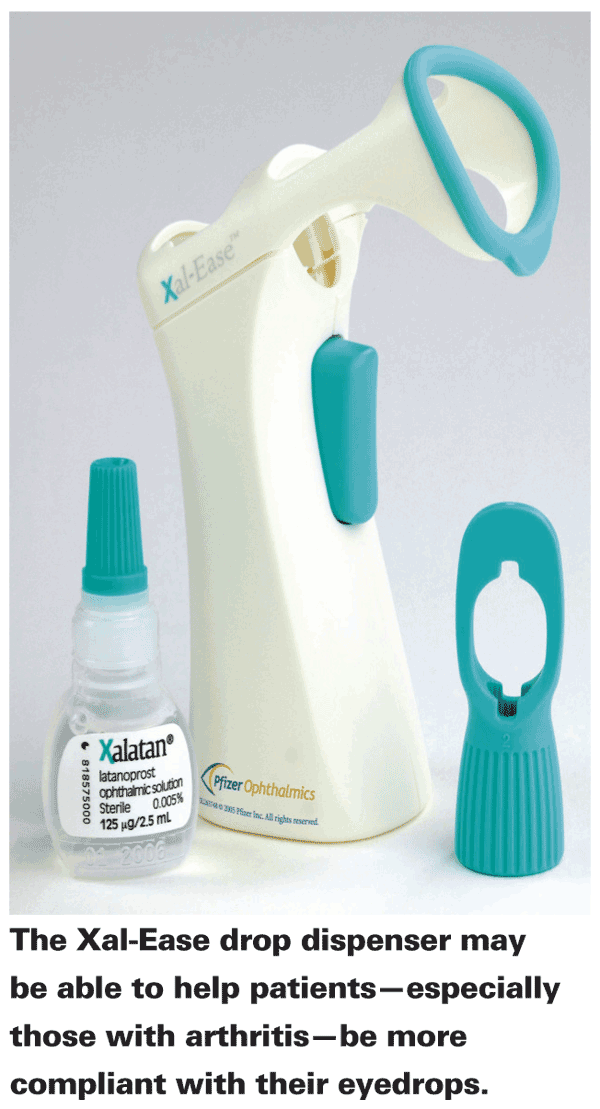
Patients can also use technology for extra compliance support. To this end, dose organizers with timers and reminder systems via text message, e-mail or smartphone are becoming more and more common. Also, you and the patient can explore different treatments and dosing regimens. Typically, compliance is inversely proportional to dosing frequency: Higher compliance generally follows from lower frequency of dosing.
If a variety of treatments are under consideration and the patient or clinician foresees that there will be some difficulty with compliance, the required regimen might become a factor in the decision process.
Beyond dosing regimen, dosing duration is important for compliance as well. Instructing the patient about the importance of using the drops for the prescribed amount of time, rather than stopping as soon as symptoms subside, is imperative for the best recovery. Especially for anti-infective agents, this duration is important in reducing microbial resistance and minimizing the risk of sequelae.
In conclusion, equipping patients with the knowledge and tools necessary for their compliance when ocular medication gives the best chance of effective treatment. Next time you hand out a medication, make sure that the patient is prepared to pursue treatment outside the office.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: Clinical applications. Jama 2002;288:22:2880-3.
2. Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol 1990;74:8:477-80.
3. Kim MS, Choi CY, Kim JM, et al. Microbial contamination of multiply used preservative-free artificial tears packed in reclosable containers. Br J Ophthalmol 2008;92:11:1518-21.
4. Henriksen TM, Breland TA. Carbon mineralization, fungal and bacterial growth, and enzyme activities as affected by contact between crop residues and soil.BiolFertil Soils 2002;35:41-8.
5. Systane website. Alcon, Inc. http://www.systane.com/Preservative-Free-Eye-Drops.aspx. Accessed 9 March 2010.
6. Acuvail [prescribing information].
7. Karkkainen TR. The effect of refrigeration on the osmolality and pH of nonpreserved artificial tears containing carboxymethylcellulose.Optom Vis Sci 2001;78:1:37-9.



