C. Robert Bernardino, MD, FACS
Thyroid eye disease, also called thyroid-related immune orbitopathy and Graves¡¯ ophthalmopathy/orbitopathy, is an autoimmune disorder, as well as the most common orbital disease, affecting 25 to 50 percent of patients with Graves¡¯ disease.1 As such, it is also one of the more common diseases in which ophthalmologists work in conjunction with other medical professionals (endocrinologists, internal medicine physicians, etc.) to deliver patient care. The clinical signs feature a combination of eyelid retraction, lid lag, proptosis, restrictive extraocular myopathy and optic neuropathy. Clinical symptoms of most patients are usually mild, consisting of ocular irritation with redness and tearing, ¡°stare¡± due to lid retraction and exophthalmos and periorbital swelling. Nevertheless, approximately 28 percent of TED cases are severe, with restricted motility leading to diplopia, exposure keratopathy and optic neuropathy.2 In this review, we will highlight some of the recent advances in care for patients with TED.
Epidemiology
A recent epidemiologic study of white American patients with TED from the Mayo Clinic determined an age-adjusted annual incidence of 16 per 100,000 for women and three per 100,000 for men.3 TED affected women six times more frequently than men (86 percent versus 14 percent of cases, respectively).
Thyroid Dysfunction
George Bartley, MD, indicated that among patients with TED in the incidence cohort, approximately 90 percent had Graves¡¯ hyperthyroidism; 1 percent had primary hypothyroidism; 3 percent had Hashimoto¡¯s thyroiditis, and 5 percent were euthyroid.4 One temporal relationship between TED and hyperthyroidism was also found. In 20 percent of TED patients, both diagnoses were made at the same time; in about 60 percent of them, TED occurred within the first year of hyperthyroidism diagnosis; the rest of TED patients had the risk of thyroid abnormalities¡ª25 percent within one year and 50 percent within five years.
Clinical Course and Follow-up
TED has variable presentations and severities in different individuals. Therefore, it is of essential importance for patients to be checked by ophthalmologists in different stages of TED. We suggested three time points for visit¡ªonset of thyroid dysfunction, acute onset of TED and stable condition of TED. Most patients with newly diagnosed thyroid dysfunction are free of clinical symptoms, though some subtle clinical signs can be found such as mild lid lag or retraction. A baseline examination or record can be established at this stage. Additionally, it facilitates the good relationship between patients and ophthalmologists. Such mutual trust will bring patients better understanding and communications with physicians, which is crucial to those patients who need surgical intervention to rescue vision in the acute stage or to achieve better cosmetic results in the stable stage of TED.
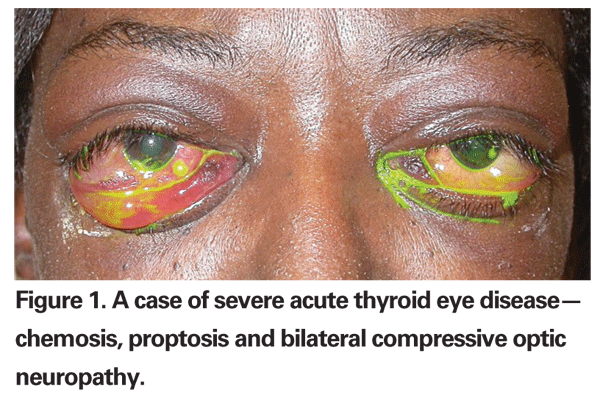
With disease progression, clinical signs present as chemosis, conjunctival injection and redness, and swollen upper and lower eyelids. If left untreated, acute onset of TED ensues. With increasing tissue swelling, impairment of extraocular muscle motility or restrictive myopathy occurs. Diplopia is usually prominent in such condition. Elevation of intraorbital pressure can lead to compressive optic neuropathy which, if left untreated, can lead to vision loss in severe TED patients. In the acute stage of TED (See Figure 1), the ophthalmologist¡¯s role is to offer medical or surgical treatments to relieve discomfort and rescue vision.
Regardless of treatment or not, TED usually moves to a stable or chronic stage as time passes. Though acute inflammation is under control, variable sequela such as proptosis, lid retraction or strabismus remain. During this stage, intervention with proper ocular surgery tends to achieve better cosmetic or visual outcomes.
Clinical Diagnosis
TED is clinically diagnosed if eyelid retraction (upper eyelid position at or above the superior corneoscleral limbus) occurs along with thyroid dysfunction (preferably included but not limited to), or extraocular muscle involvement (determined by CT, MRI or ultrasonography), or exophthalmos (exophthalmometry measurement ¡Ý20 mm), or optic neuropathy. In the absence of eyelid retraction, TED can be considered to be present only if extraocular myopathy, optic neuropathy or exophthalmos exists simultaneously with thyroid dysfunction.
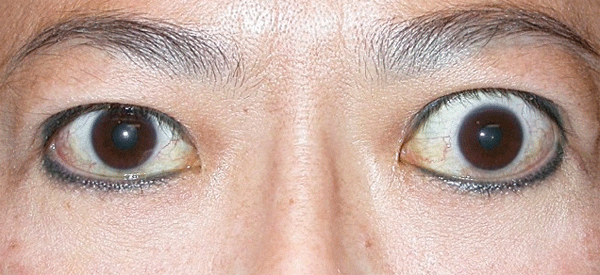
Image Studies
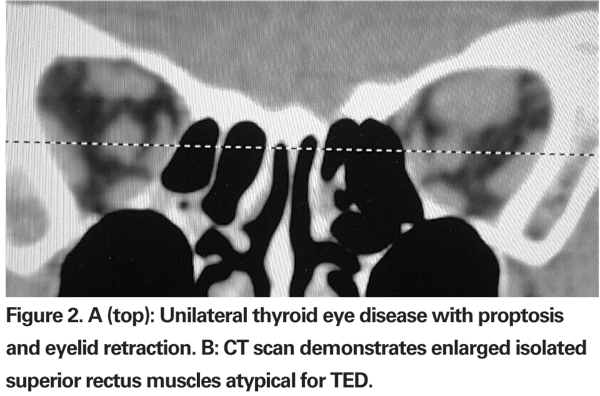
As mentioned before, TED is mainly a clinical diagnosis. Image studies play some role when the diagnosis is uncertain. CT is used to assess EOMs enlargement, fatty expansion and optic nerve compression, especially before surgery and as a follow-up after treatment. Compared to CT, MRI does not offer significant advantages, except for evaluation of the optic nerve.5 Typically, enlargement of the EOMs involves the belly of the muscle and spares the anterior tendinous insertions; this is in contrast to orbital pseudotumor, in which muscle tendon insertion tends to be thickened. By image studies, inferior rectus is the most commonly involved extraocular muscle, followed by medial rectus, superior rectus and lateral rectus. In cases where an isolated lateral or superior rectus is enlarged causing proptosis, a diagnosis other than TED should be entertained, leading to muscle biopsy (See Figures 2a and b).
In addition to traditional imaging, digital infrared thermal imaging may aid in determining the inflammatory state of TED and follow-up effects after disease treatment.6 This imaging modality can detect local temperatures of orbit, and that these temperatures are elevated in active TED. The temperature decreased in patients who were responsive to methylprednisolone pulse therapy.
Management
An association between tobacco smoking and TED was first described in 1987,7 and there are growing numbers of studies to support a causal link.8 These studies provide strong evidence for a causal association between smoking and development of TED. Current smokers were also more likely to experience disease progression or poorer outcome of treatment. Thus, TED patients who are smokers should be given strong support to quit smoking.
Permanent control of hyperthyroidism can be achieved by either radioiodine or thyroidectomy, both of which seem to be essential for the course of TED. However, Luigi Bartalena found that radioiodine therapy alone will cause eye disease progression in TED patients with high-risk traits.9 These traits include smoking, severe hyperthyroidism, uncontrolled hypothyroidism, high level of TSH receptor antibody and other preexisting eye inflammatory disease. However, such exacerbation by radioiodine can be prevented with concomitant glucocorticoid administration. Thyroidectomy itself does not worsen the TED.10
Concerning ophthalmopathy of TED, there are three main managements for intervention¡ªmedication, radiation and surgery. Corticosteroids are the mainstay of anti-inflammatory and immunosuppressive therapy of TED and are usually the first choice for treatment. On account of the side effects of steroids, they are not administrated unless moderate to severe symptoms of active orbital inflammation (such as severe orbital congestion, corneal involvement or optic neuropathy) are present. Unfortunately, disease recurrence after cessation of treatment is not uncommon.
Several novel immunomodulatory medications were used as TED therapy. Rapamycin, a macrolide-class antibiotic, also inhibits cytokines and growth factor-mediated proliferation of fibroblasts and immune cells (T and B cells). Rituximab is a monoclonal antibody directed against CD20, a B lymphocyte surface antigen. Inhibition of B cells should theoretically eventually dampen the inflammatory process in TED. Infliximab is a monoclonal antibody against TNF-a, a cytokine, which is of known importance in TED. All of these drugs have demonstrated effectiveness in select cases of steroid-resistant TED.
Radiotherapy is effective in TED because inflammatory cells and lymphocytes, in particular, are radiosensitive. Results are typically seen within one to eight weeks of treatment. Colum Gorman, MD, PhD, reported that radiation lessens the amount of proptosis, and this may, indeed, be true;11 however, the effect seems to be less apparent after three months of radiotherapy. Severe complications such as radiation retinopathy with vision loss from this low-dose radiation are uncommon. The most common complication is dry eye, the symptoms of which can be lessened with some lubricating eye drops.
The common indications for surgical intervention in TED include optic neuropathy, diplopia, corneal exposure and cosmesis. By and large, surgical procedures may be directed toward orbital decompression, strabismus repair, and the correction of eyelid malpositions. As a general rule, the most invasive surgery is performed first; thus, optic decompression, strabismus surgery, eyelid surgery is the exact temporal sequence.
Orbital decompression may be necessary on an urgent basis for apical compression of the optic nerve or severe proptosis with corneal ulceration refractory to other treatments. Decompression of orbital contents can be accomplished either by removing bony walls of the orbit, allowing prolapse of the orbital contents, or by removing orbital fat, thereby decreasing the amount of tissue in the orbit. Any of the walls of the orbit may be removed, allowing expansion into the ethmoid sinus medially, maxillary sinus inferiorly, the temporal fossa laterally, and the cranial cavity superiorly. Removal of orbital fat without bony resection is now performed as a primary procedure in selected patients, particularly those with CT evidence of orbital fat expansion dominant instead of extraocular muscle enlargement.
It¡¯s not uncommon that strabismus is worsened after decompression surgery. Due to this reason, strabismus surgery is best performed after decompression, when the ocular deviation has stabilized, usually at least six months.
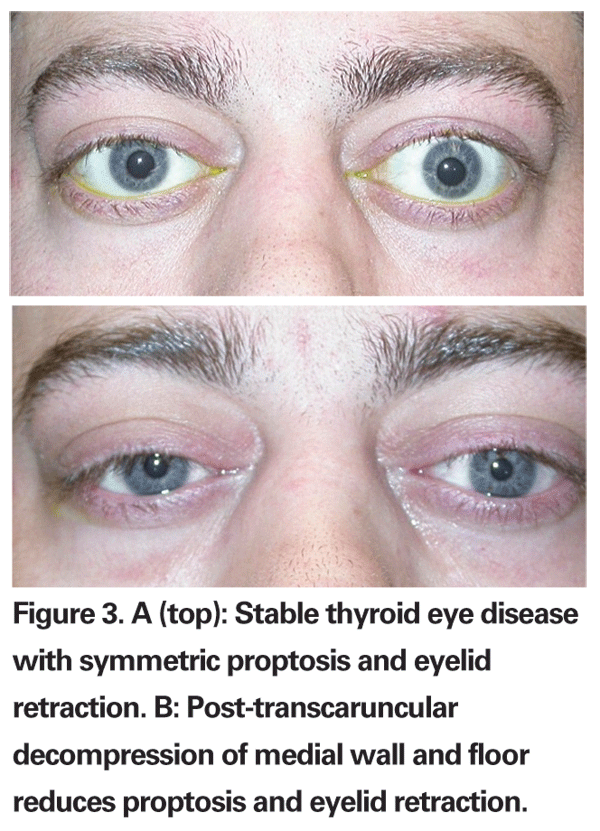
Because vertical muscle surgery can result in further lid retraction secondary to connective tissue attachments between these muscles and the lid musculature, lid surgery is best performed after strabismus surgery. Further decompression alone can often reduce lid retraction (See Figure 3a and b). Common eyelid procedures include lateral tarsorrhaphy, lengthening of M¨¹ller¡¯s and levator muscles, lower lid elevation and blepharoplasty. Lid surgery may help improve cosmesis and may often be combined with vertical eyelid repositioning. As a general rule, most oculoplastic surgeons prefer to wait until orbitopathy is stable for at least six months, with no change in lid position or eyelid edema.
Dr. Bernardino is at the Yale University School of Medicine, Department of Ophthalmology, Section of Ophthalmic Plastics and Orbital Surgery. Dr. Hsiao is from the
1. Garrity JA, Bahn RS. Pathogenesis of
2. Prummel M, Bakker A, Wiersinga W,
3. Bartley GB, Fatourechi V, Kadrmas EF, et al. The incidence of Graves¡¯ ophthalmopathy in
4. Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in
5. Enzmann D, Marshall WH, Rosenthal AR. Computed tomography in
6. Chang TC, Hsiao YL, Liao SL. Application of digital infrared thermal imaging in determining inflammatory state and follow-up effect of methylprednisolone pulse therapy in patients with Graves¡¯ ophthalmopathy. Graefe¡¯s Arch Clin Exp Ophthalmol 2008;246:45-9.
7. Hagg E, Asplund K. Is endocrine ophthalmopathy related to smoking. BMJ 1987;295:634-5.
8. Krassas GE, Wiersinga W. Smoking and autoimmune thyroid disease: The plot thickens. Eur J Endocrinol 2006;154 777-80.
9. Bartalena L, Marcocci C, Pinchera A.On the effects of radioiodine therapy on
10. Wiersinga M, Bartalena L. Epidemiology and prevention of
11. Gorman CA, Garrity JA, Fatourechi V. A prospective, randomised, double-blind, placebo controlled study of radiotherapy for



