This installment of a two-part Retinal Insider will look at the considerations for diagnostic vitrectomy in patients with uveitis, with next month's column addressing therapeutic vitrectomy.
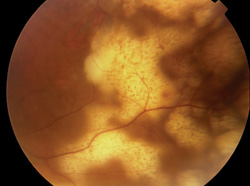 |
| Figure 1. Subretinal infiltration in a patient with primary intraocular lymphoma. |
Indications
In the vast majority of cases, the diagnosis of posterior segment inflammatory disease may be ascertained through the combination of a comprehensive medical and ophthalmic history, review of systems, complete ocular examination, and directed laboratory investigations. Oftentimes, the diagnosis can be made on the clinical appearance alone within the correct clinical context. For example, toxoplasmic retinochoroiditis in an otherwise healthy patient presenting with an area of focal retinitis adjacent to a hyperpigmented scar with accompanying vitritis, or that of cytomegalovirus (CMV) retinitis in a profoundly immunosuppressed HIV/AIDS patient with a typical wedge of hemorrhagic retinitis and scant vitritis.
Diagnostic dilemmas arise in situations where the clinical presentation is obfuscated by media opacity or is atypical, when the systemic workup is inconclusive or where there has been an inadequate response to conventional therapy. In such cases, vitreoretinal surgical techniques to obtain vitreous, retinal, subretinal or chorioretinal biopsy specimens for laboratory analysis are essential diagnositic tools in differentiating between purely inflammatory, infectious and neoplastic etiologies, and allowing the commencement of appropriate therapy (See Table 1). Specifically, diagnostic vitrectomy is employed in the setting of suspected infectious posterior uveitis due to bacteria (Propionibacterium acnes delayed onset endophthalmitis), viruses (the herpetic necrotizing retinitides, acute retinal necrosis, or ARN, and progressive outer retinal necrosis syndromes, or PORN), protazoal and helminthic diseases (Toxoplasmosa gondii and Toxocara spp.), and fungi. In fact, vitreous biopsy is the only means by which primary intraocular lymphoma and intraocular Whipple's disease may be definitively diagnosed.1 Other non-infectious entities for which vitreous, subretinal or retino-choroidal tissue sampling may be diagnostic include the neoplastic masquerade syndromes of primary or secondary intraocular lymphoma, metastatic disease and choroidal melanoma, as well as atypical presentations of ocular sarcoidosis.
Surgical Techniques
Vitreous biopsy techniques include a one-port approach using either a 22- or 25-ga. needle2 or the newly developed 23-ga. hand-held portable vitrectomy unit (e.g., Visitrec, BD Ophthalmics). Advantages to this approach include the convenience of the outpatient setting and need for minimal equipment. So, it may be ideally suited for cases in which a small sample volumes are required, such as that of a vitreous tap and inject procedure for acute postoperative endophthalmitis, or when exclusion or confirmation of only one diagnostic entitiy (e.g. ARN) is required. Disadvantages include smaller sample volumes and the potential for complications associated with vitreous base traction and hypotony.
A standard three-port vitrectomy is generally preferred. It allows larger sample volumes to be obtained in a controlled fashion, greater latitude in the scope of laboratory testing and the possibility of simultaneous therapeutic vitrectomy, if needed. New 25-ga., transconjunctival, sutureless vitrectomy systems may be ideally suited for diagnostic purposes or when a limited core vitrectomy to clear the visual axis is required.
malignancy
management of uveitis |
An undiluted, pure vitreous sample is obtained for microbiology, cyto-pathology, antibody studies with matched serum samples, and for polymerase chain reaction (PCR) analysis. Up to 1 ml of specimen may be collected by cutting and manually aspirating the vitreous into a 3-ml syringe connected to the vitrector with the infusion off. A dilute specimen may then be obtained with the infusion on, manually aspirating into a 20-cc syringe or by collecting the vitreous wash from the machine cassette. The cassette, in turn, may be divided into two aliquots; one for flow cytometry and immunohistochemistry and a second for microbiology, culture and special stains as indicated.
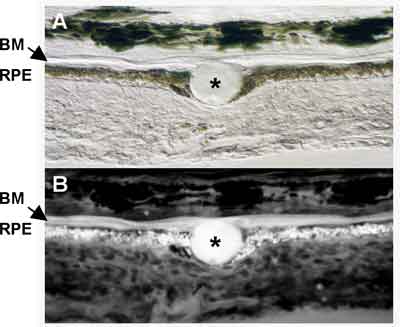 |
| Figure 2. Papanicolaou stain showing an irregular nuclear outline, course chromatin pattern and prominent nucleoli in primary intraocular lymphoma. |
Occasionally, analysis of the vitreous is either inappropriate or fails to provide useful diagnostic information. Certain infectious processes (i.e., atypical presentations of toxoplasmosis, necrotizing herpetic retinitis, or Candida retinitis) or non-infectious masquerades (i.e., retinal sarcoidosis or intraocular lymphoma presenting with sub-RPE infiltration) which are primarily localized to the neurosensory retina or RPE may require endoretinal3,4 or subretinal5 biopsy for definitive diagnosis (See Figure 1). In other instances, chorioretinal biopsy may be required for patients with progressive, medically unresponsive, sight-threatening chorioretinal lesions.6 The procedures themselves are described in detail elsewhere3-6 with the ideal biopsy site for each being somewhat limited by the surgical anatomy; lesions anterior to the equator are more suited to chorioretinal biopsy while those in a more posterior location are more accessible to endoretinal incision techniques. Chorioretinal biopsy allows a larger tissue sample to be obtained with preservation of the anatomic relationships between the retina and choroid but also carries not inconsiderable risk of intraoperative and postoperative complications. With either technique, the biopsy specimen should be taken from the border of inflamed and uninvolved tissue to increase the possibility of identifying bona fide pathology. Tissue processing is also similar for each procedure with the specimen being divided into three sections on the OR: one section frozen for immunopathology; a second placed in 4% glutaraldehyde for light and electron microscopy; and a third for microbiology, culture and PCR.
Diagnostic Techniques—Cytology
Preoperative communication with respective laboratories is essential for effective intraocular sample processing. Cytological evaluation requires immediate attention to prevent cellular degradation, especially in cases of suspected intraocular lymphoma, where rapid transport to the lab in tissue-culture medium (e.g., RPMI-1640S) may preserve cellular viability. While vitreous cytopathology remains the gold standard for the diagnosis of intraocular lymphoma, its sensitivity is low.7,8 The samples are typically paucicellular, and interpretation may vary depending on the expertise of the cytopathologist (See Figure 2). To improve the diagnositic yields, cells with cytologic abnormalities may be isolated by laser capture or manual microdissection for PCR-based molecular assays to detect IgH, bcl-2, or T-cell receptor gamma gene rearrangements.7,8,9
Immunohistochemistry
Immunohistochemical techniques are employed to detect cells of tissue-bound antigens with monoclonal antibodies, either by microscopic examination of immunoflourescence or immunoperoxidase-stained slides or by using fluorescence-activated cell sorters, otherwise known as flow cytometry (FCI). Both of these techniques permit the immunophenotyping of lymphocytes and so, have been applied to the diagnosis of intraocular lymphoma and its differentiation from infectious and non-infectious uveitis.10,11
Specifically, most primary intraocular lymphomas consist of populations of monoclonal B-lymphocytes that stain for specific B-cell markers (CD-19, CD-20, and CD-22) and have restricted expression of kappa or lambda chains. In non-infectious posterior uveitis, there is a predominance of CD4+ helper or inducer T-lymphocytes and elevated interleukin-2 receptor levels (CD-25) which is correlated with uveitis activity.12
In one study, FCI identified intraocular lymphoma in seven of 10 patients as compared to only three diagnosed by cytology.11 In another, FCI provided corroborative support in six patients diagnosed by both modalities.13 Most recently, researchers at Bascom Palmer14 have reported that CD-22+ B-lymphocytes comprising >20 percent of total cells on FCI had a positive predictive value of 88 percent for lymphoma while a CD4:CD8 T-lymphocyte ratio of greater than 4 had a similarly positive predictive value of 70 percent for immunologically mediated uveitis.
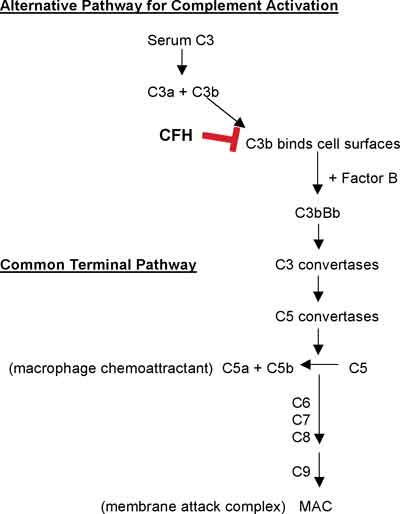 |
| Figure 3. Gram stain revealing a colony of gram-positive rods consistent with P. acnes. Note the yellow lens capsule inferiorly. |
Cytokine Analysis
Cytokine analysis of vitreous samples from patients with suspected intraocular lymphoma may prove to be a useful adjunct in distinguishing this entity from inflammatory posterior uveitis. Interleukin-10 (IL-10) is preferentially produced by malignant B-lymphocytes in patients with intraocular lymphoma, whereas, interleukin-6 (IL-6) is found in high levels in patients with inflammatory uveitis.15 Specifically, elevated relative ratios of IL-10 to IL-6 were found in 24 or 31 ocular lymphoma cases, supporting the diagnosis of lymphoma.16
Culture
While culture remains the gold standard for the diagnosis of intraocular infection, especially in cases of bacterial endophthalmitis, many intraocular microbes (viruses) are difficult to recover and identify by this method. It is important to hold bacterial specimens for a least one week and fungal cultures for one month as some organisms (Propionibacterium acnes) may require extended time periods to grow (See Figure 3).
Intraocular Antibody Analysis
Intraocular antibody production as a measure to the host response to a specific microbial pathogen can be computed utilizing the Witmer quotient: the ratio of specific antibody (aqueous or vitreous)/total IgG (aqueous or vitreous) to specific antibody (serum)/total IgG (serum) as measured by enzyme-linked immunosorbant assay (ELISA) or radioimmunoassay.17 A ratio of greater than 4 is considered diagnostic of local antibody production.18 Antibody testing of ocular fluids remains the gold standard for the diagnosis of ocular toxocarasis.19 It has been used more widely in Europe than in the United States as an adjunct to the diagnosis of toxoplasmosis,20 necrotizing herpetic retinitis due to herpes simplex virus (HSV) and varicella zoster virus (VZV) while it is of little value in the diagnosis of CMV retinitis.21
PCR
PCR is a highly sensitive and specific assay that has been applied to the identification of a wide variety of intraocular ocular pathogens22,23 (See Table 2). It is most valuable clinically when the differential diagnosis has been well-defined, and a diagnostic dilemma remains. For example, in differentiating among the various causes of necrotizing viral retinitis and from that caused by ocular toxoplasmosis. In a series of 38 eyes of 37 patients with "diagnostic dilemmas" thought to be due to intraocular infection, CMV, HSV and VZV was detected in 24 cases by PCR analysis of either aqueous or vitreous tap specimens.24 Significantly, none of the remaining PCR negative cases had a clinical course consistent with a viral retinitis. While the sensitivity of PCR for the diagnosis of ocular toxoplasmosis is only about 60 percent from vitreous specimens,25 PCR seems to be complementary to the Witmer coefficient calculation of intraocular antibody production, with one of the two assays (but not both) being positive.26 In many cases of necrotizing retinitis, PCR and/or antibody determinations from the aqueous alone may provide sufficient substrate for analysis, obviating the need for vitrectomy.27
–Toxoplasma gondii –Oncocerca volvulus
–Candida albicans – Aspergillus spp. |
PCR-based assays have also been developed for the detection of bacteria and fungi in cases of both acute, postoperative and delayed-onset endophthalmitis. In one study using "universal" 16 S rDNA primers, bacterial DNA was amplified in nearly all cases of acute postoperative endophthalmitis,28 while in the Endophthalmitis Vitrectomy Study, the reported rate of culture positive cases was only 70 percent.29 Similarly, diagnostic yields of up to 92 percent in cases of delayed-onset endophthalmitis due to Propionibacterium acnes, Staphlococcus epidermidis, or Actinomyces israelii 30 and fungi31 have been reported, significantly improving the time to diagnosis over traditional techniques.
Finally, PCR screening of vitreous samples is invaluable in the diagnosis of medically unresponsive, atypical or otherwise unusual causes of posterior uveitis, such as suspected Whipple's disease,1 Lyme disease,32 ocular tuberculosis, 33 or cat-scratch disease.34
Diagnostic Yield
The overall diagnostic yield of vitrectomy in eyes with suspected posterior segment infection or malignancy was 39 percent in a series of 87 patients.35 Intraocular antibody testing and PCR had the highest positive yields at 46 percent and 39 percent, respectively. A specific diagnosis was reached more often (42 percent of 65 eyes) when an underlying infection was suspected preoperatively, in contrast to that of intraocular malignancy (10 percent of 71 eyes).
Most recently, vitreous analysis led to a diagnosis in 61.5 percent in 78 consecutive patients in the earlier cited Bascom Palmer study14 with 81.6 percent of patients having a final diagnosis that matched their indication for surgery. When the initial diagnosis was compared to the final clinical diagnosis, the efficiency of the diagnostic procedure of cytologic evaluation, flow cytometry and bacterial/fungal culture was 67 percent, 79 percent and 96 percent, respectively. The positive predictive value of cytologic evaluation for lymphoma was 100 percent, while the negative predictive value was 60.9 percent.
For infection, the positive predictive value of bacterial/fungal culture was 100 percent, and the negative predictive value 94.9 percent. These studies demonstrate that diagnostic vitrectomy with directed vitreous fluid analysis is an effective strategy in differentiating between intraocular lymphoma, chronic intraocular infections and atypical chorioretinitis, allowing the prompt initiation of appropriate therapy with greater confidence.
Dr. Vitale is chief of the Uveitis Service and a member of the Vitreoretinal Division at the John A. Moran Eye Center, University of Utah. He is a consultant to Bausch & Lomb. Contact him at Albert.Vitale@hsc.utah.edu.
1. Rickman LS, Freeman WR, Green WR, Feldman ST, et al. Brief report: uveitis caused by Tropheryma whippelii (Whipple"s bacillus). N Engl J Med 1995;332:363-366.
2. Lobo A, Lightman S. Vitreous aspiration needle tap in the diagnosis of intraocular inflammation. Ophthalmology 2003;110:595-599.
3. Freeman WR, Wiley CA, Gross JG, Thomas EL, et al. Endoretinal biopsy in immunosuppressed and healthy patients with retinitis. Indications, utility, and techniques. Ophthalmology 1989;96:1559-65.
4. Rutzen AR, Ortega-Larrocea G, Dugel PU, Chong LP, et al. Clinicopathologic study of retinal and choroidal biopsies in intraocular inflammation. Am J Ophthalmol 1995;119:597-611.
5. Levy-Clarke GA, Byrnes GA, Buggage RR, Shen DF, et al. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a subretinal lesion. Retina 2001;21:281-4.
6. Martin DF, Chan CC, de Smet MD et al. The role of chorioretinal biopsy in the management of posterior uveitis. Ophthalmology 1993;100:705-714.
7. Baehring JM, Androudi S, Longtine JJ, Betensky RA, et al. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer 2005;104:591-597.
8. Davis JL. Diagnosis of intraocular lymphoma. Ocular Immunology & Inflammation 2004;12:7-16.
9. Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control 2004;11:285-295.
10. Davis JL, Solomon D, Nussenblatt RB, Palestine AG, et al. Immunocytochemical staining of vitreous cells. Indications, techniques, and results. Ophthalmology 1992;99:250-256.
11. Davis JL, Viciana AL, Ruiz P. Diagnosis of intraocular lymphoma by flow cytometry. Am J Ophthalmol. 1997;124:362-372.
12. Deschenes J, Char DH, Freeman W, Nozik R, et al. Uveitis: lymphocyte subpopulation studies. Trans Ophthalmol Soc UK 1986;105:246-251.
13. Zaldivar RA, Martin DF, Holden JT, Grossiklaus HE. Primary intraocular lymphoma: clinical, cytologic, and flow cytometric analysis. Ophthalmology 2004;111:1762-7.
14. Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol 2005;140:822-829.
15. Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol 1995;120:671-673.
16. Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc 2003;101:175-292.
17. Witmer RH. Antibody formation in rabbit eye studied with fluorescein-labeled antibody. Am Arch Ophthalmol 1955;53:811-816.
18. Dussaix E, Cerqueti PM, Pontet F, Bloch ME. New approaches to the detection of locally produced antiviral antibodies in the aqueous of patients with endogenous uveitis. Ophthalmologica 1987;194:145-149.
19. Benitez del Castillo JM, Herreros G, Guillen JL, Fenoy S, et al. Bilateral ocular toxocariasis demonstrated by aqueous humor enzyme-linked immunosorbent assay. Am J Ophthalmol 1995;119:514-516.
20. Ronday MJ, Ongkosuwito JV, Rothova A, Kijlstra A. Intraocular angi-Toxoplasma gondii IgA antibody production in patients with ocular toxoplasmosis. Am J Ophthalmol 1999;127:294-300.
21. de Boer, JH, Verhagen C, Bruinenberg M, Rothova A, et al. Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol. 1996;121:650-658.
22. Bodaghi B, LeHoang P. Testing ocular fluids in uveitis. Ophthalmol Clin N Am 2002;15:271-279.
23. Van Gelder RN. CME review: polymerase chain reaction diagnosis for posterior segment disease. Retina 2003;23:445-452.
24. Knox CM, Chandler D, Short GA, Margolis TP. Polymerase chain reaction-based assays of vitreous samples for the diagnosis of viral retinitis. Use in diagnostic dilemmas. Ophthalmology. 1998;105:37-44; discussion 44-45.
25. Montoya JG, Parmley S, Liesenfield O, Jaffe GJ, Remington JS. Use of the polymerase chain reactions for diagnosis of ocular toxoplasmosis. Ophthalmology 1999;106:1554-1563.
26. Fardeau C, Romand S, Rao NA, Cassoux N, et al. Diagnosis of toxoplasmic retinochoroiditis with atypical clinical features. Am J Ophthalmol 2002;134:196-203.
27. Tran TH, Rozenberg F, Cassoux N, Rao N, et al. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol 2003;87:79-83.
28. Lohmann CP, Heeb M, Linde HJ, Gabel VP, et al. Diagnosis of infectious endophthalmitis after cataract surgery by polymerase chain reaction. J Cataract Refract Surg 1998;24:821-826.
29. Lohmann CP, Linde HJ, Reischl U. Improved detection of microorganisms by polymerase chain reaction in delayed endophthalmitis after cataract surgery. Ophthalmology 2000;107:1047-1051.
30. Endophthalmitis Vitrectomy Study. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 1996;122:830-846.
31. Anand A, Madhavan H, Neelam V, Lily T. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology 2001;108:326-330.
32. Mikkila H, Karma A, Viljanen M, Seppala I. The laboratory diagnosis of ocular Lyme borreliosis. Graefes Arch Clin Exp Ophthalmol 1999;237:225-30.
33. Ortegea-Larroca G, Bobadilla-del-Valle M, Ponce-de-Leon A, Sifuentes-Osomio J. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Arch Med Res 2003;34:116-119.
34. Kerkhoff FT, Bergmans AM, van Der Zee A, Rothova A. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol 1999;37:4034-4038.
35. Mruthyunjaya P, Jumper JM, McCallum R, Patel DJ, Cox TA, Jaffe GJ. Diagnostic yield of vitrectomy in eyes with suspected posterior segment infection or malignancy. Ophthalmology 2002;109:1123-1129.



