Late Friday afternoon finds an urgent, disgruntled patient at our doorstep. His post-LASIK interface haze and vision had worsened despite aggressive topical and systemic steroid therapy, suggesting a stromal infection. Staphylococcal or mycobacterium chelonei interface infection enters my differential. At moments like this we surgeons hope that newer, better antibiotic prophylaxis will provide true protection against potentially blinding postoperative infections.
Despite their common use in clinical practice, it has been exceedingly difficult to establish the efficacy of modern topical antibiotics unequivocally as preventive agents in our crusade for complication-free elective surgery. There is no squeaky-clean prospective, randomized clinical study. The low incidence of blinding corneal and vitreous disease means that statistical significance would require exorbitantly expensive trials with thousands of subjects. Thus, in the best interest of our patients, we decide what is truly best for surgical candidates within the context of our own practice. We remain vigilant for vision-threatening infections, with bacterial keratitis and endophthalmitis the most emergent.
Perioperative Antibiotics?
Most surgeons agree that perioperative antibiotics can prevent postoperative infections. A 1974 study reported a remarkable 23-fold decrease in postoperative endophthalmitis following extracapsular cataract surgery when perioperative topical chloramphenicol was prescribed.1 This landmark study truly convinced most surgeons that antibiotics were necessary for all of their elective surgery patients. Prospective studies have been few and far between.
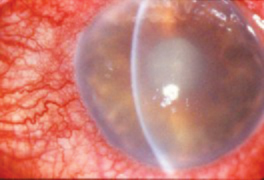 |
| Acute hypopyon indicative of severe postoperative inflammation. Bacterial endophthalmitis is the diagnosis of exclusion. |
Data on thousands of postoperative cataract surgery patients in the phacoemulsification era demonstrate a trend towards endophthalmitis prevention with intraoperative irrigating solution antibiotic administration.2,3
Another showed that the only demonstrably effective method for endophthalmitis prevention in the phacoemulsification population was the traditional preoperative povidone iodide scrub.4 Nevertheless, an emerging trend towards increased incidence of endophthalmitis with clear corneal incisions strongly implicates a role for prophylactic topical antimicrobials.
Reversing the Tide
Recently, a retrospective review of more than 9,000 phacoemulsification patients at Utah's Moran Eye Center revealed a significant three-fold higher rate of endophthalmitis in patients treated with perioperative ciprofloxacin drops when compared to those treated with topical ofloxacin.5 A Japanese study found a six-fold increase in endophthalmitis with the switch from scleral tunnel to clear corneal incisions.6
This data also suggests that continuously therapeutic aqueous levels of antimicrobial are important in controlling bacterial introduction into the anterior chamber after surgery has been completed, likely through paroxysmal communications between the anterior chamber and the ocular surface through a sutureless temporal corneal incision. Thus, although sterilization of the ocular surface and meticulous sterile technique are clearly required of the cataract surgeon, the role of topical antibiotics has not definitively been proven essential.
This proof would be prohibitively expensive considering the costs of prospective randomized clinical trials, and the exceedingly rare incidence of endophthalmitis. Nonetheless, there is alarming new data suggesting that the risk of postoperative endophthalmitis has risen, perhaps as a result of the significant move to clear corneal temporal incision by many surgeons.
The Next Generation
Today's ophthalmic media teem with reports of new generation topical ophthalmic antibiotics. Clinicians anticipate even better protection with even less toxicity. Despite remarkable improvements in the spectrum and efficacy of systemic antibiotics, these innovations may not necessarily lead to benefits for patients at risk for ocular infection.
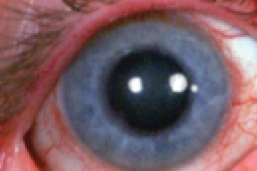 |
| Acute anterior segment inflammation consistent with severe postoperative bacterial infection. |
As a result of these introductions as well as patent expirations, ofloxacin 0.3% (Ocuflox, Allergan) and Ciloxan 0.3% (Ciloxan, Alcon) will become available as generic preparations, undoubtedly raising important questions about such issues as vehicle quality, preservatives, efficacy and cost savings.
With the development of newer fluoroquinolones, a broader spectrum of activity is assumed to provide superior protection. Clearly, the newer drugs have significantly lower Mean Inhibitory Concentrations for gram-positive organisms as well as some additional classes of pathogens, including mycobacteria, chlamydia and anerobes. Gram-negative activity, however, has remained universally vigorous among all of the available topical fluoroquinolones. As resistance develops to currently popular agents, treatment failures and even prophylaxis failures will ensue. Because the majority of emergent ocular infections are gram-positive, particularly postoperative endophthalmitis, the enhanced coverage and broadened spectrum are welcomed.
Solubility and Penetration
Ocular surface, intracameral and serum antibiotic levels and Mean Inhibitory Concentrations can be confused. MICs are established by the National Committee of Clinical Laboratory Standards and are derived from in vitro data obtained under strictly standardized conditions. These standards are based upon concentrations of antibiotic in the serum: There are no standards for topical ocular therapy that represent the concentrations of antibiotics in ocular tissues. Thus, ocular surface concentrations from a single drop may exceed the maximum achievable serum concentration by several fold.
Further, interactions between the tear film, the ocular surface and sustained release capacitance effects created by absorbed drug in the conjunctiva and uvea may also affect peak and trough bioavailability. Surface kill curves may be more relevant to conjunctivitis or preop sterilization, and intrastromal corneal levels more applicable to refractive surgery, while aqueous humor levels may be more important during postop cataract healing. These levels may differ in potency from tissue to tissue, from drug to drug, and from bug to bug. As a result, detailed analyses of target tissue bioavailability with respect to each pathogen is necessary in order to make any conclusions about the best fluoroquinolone for a given indication.
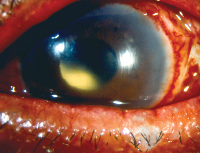 |
| Increased incidence of endophthalmitis with clear corneal incisions implicates a role for prophylactic topical antimicrobials. |
Many orally administered fluoroquinolones can provide effective intravitreal concentrations, even in the absence of inflammation. Sparfloxacin, trovafloxacin, gatifloxacin,7 moxifloxacin and levofloxacin8 all reach effective MICs within the vitreous when given by mouth. No human studies, however, have evaluated this strategy for either the prevention or treatment of endophthalmitis.9
It is accepted that ocular surface and lid organisms cause the lion's share of postop endophthalmitis. It is also likely that organisms can be introduced intracamerally not only at the time of surgery, but also afterwards through the cataract wound, particularly with clear-corneal incisions. Thus, antibiotics with rapid kill curves but poor penetration into the aqueous humor will only prevent intraoperative inoculation of surface flora. Topical agents with excellent solubility and penetration will also prevent postoperative contamination of the anterior chamber by providing continuous inhibitory concentrations despite momentary gaping of the unhealed wound due to rubbing, blinking, or unusual nocturnal positions. Once the wound has sealed, certainly by postoperative day10, the risk of inoculation should decrease markedly.
Comparative data indicating effective intraocular MICs following topical administration of levofloxacin suggests that highly soluble molecules achieve effective intracameral concentrations.10 Thus, an agent with higher, albeit effective, MICs on the ocular surface may in fact achieve much lower concentrations, below the effective MICs in the eye compared to a more soluble agent with lower MICs on the surface. So has been the case of ciprofloxacin drops compared to more soluble, commercially available agents.
This phenomenon of potency at the intended target tissue is described by the Inhibitory Quotient, or IQ: the ultimate concentration of antibiotic at the site of action. The IQ = Concentration of drug at active site ÷ MIC90. Thus an adequate IQ is equal to or greater than 1. Although directly comparative human studies are only now in progress with the recent approval and release of topical gatifloxacin and moxifloxacin, initial animal data demonstrates the superior penetration and aqueous concentrations attained with topical levofloxacin compared to gatifloxacin, ofloxacin and ciprofloxacin.11
The Nomenclature Problem
Unfortunately, considerable confusion remains regarding the current generation nomenclature system, particularly for fluoroquinolones. Most consider nalidixic acid, first synthesized in 1962, to represent the first generation, even though it is not a fluorinated molecule. Ofloxacin and ciprofloxacin are generally considered to be second generation. Thereafter, opinions vary. Several articles describe a reasonable criterion for classification, but prove contradictory with one another.
There are several ways to classify antibiotics, the most commonly accepted being spectrum of anti-microbial activity, as utilized with the cephalosporins. This system of course relies upon in vitro data. Another viable method utilizes the chemical structure or more specifically, the molecular structural activity relationships, relying upon chemical data. Finally, variations of this theme might include both characteristics as well as penetration into selected body compartments or tissues, thereby relying upon clinical efficacy data.
The nomenclature for antibiotic generation assignment clearly falls within the realm of systemic applications, and has never been determined by ophthalmology. Thus, our specialty is dependent upon the wisdom of our infectious disease and pharmacology colleagues to provide us with useful classification guidelines. An authoritative text dedicated to fluoroquinolones12 describes moxifloxacin as a fourth generation antibiotic, with gatifloxacin as a third generation and levofloxacin as a second-generation fluoroquinolone. I recall separating fluoroquinolones into generations in a manner similar to the cephalosporin generations, based upon broadened spectrum of action, while acknowledging that the system of nomenclature remains clearly arbitrary. The patent for moxifloxacin, interestingly enough, deems it a third-generation drug. More confusion to decipher.
Some sources describe both gatifloxacin and moxifloxacin as fourth-generation fluoroquinolones, while others classify them both with levofloxacin as third generation, with trovafloxacin deemed the only agent with sufficient spectrum of action worthy of the fourth-generation moniker.13
Resistance in the Exam Lane
The bottom line, regardless of nomenclature synthesized in the universe of human imagination, is killing pathogens. Since the time of sulfonamides and Alexander Fleming, antibiotic resistance has proven itself a universal nemesis. Physicians prescribing systemic antibiotics are the chief creative engine behind antibiotic resistance, particularly in congested, crowded or high pathology environments like nursing homes or intensive care units. Improper use by patients or erroneous prescriptions by doctors also contribute.
In addition, over-the-counter availability of numerous antibiotics in countries without vigorous prescription regulation also contributes to resistance, as does massive use by the agriculture and veterinarian industries. Topical ocular use is unlikely, however, to contribute to the overall worldwide problem with antibiotic resistance, due to the relatively miniscule numbers of organisms exposed on the ocular surface.
Some antibiotic classes are more likely to allow antibiotic resistance to develop. Pneumococci, for example, become resistant to fluoroquinolones and macrolides more rapidly than to ceftriaxone, a third-generation cephalosporin, in an in vitro model.14 Whether this is applicable to clinical situations, let alone ocular disease, remains to be established.
Nevertheless, eye-care professionals prescribing antibiotics should be aware that fluoroquinolone resistance developing in the community, and furthermore, that improper topical administration of antibiotics can create resistant flora. Thus, less than q.i.d. dosing, administration lasting less than the recommended seven days or longer than three weeks, and dilution with other concomitant medications can create resistance during treatment for conjunctivitis or prior to surgery. Sound advice, therefore, would include switching antibiotics prior to elective surgery if either a resistant organism is identified upon preoperative culture, or if the particular patient was known to have self-administered the intended prophylactic antibiotic improperly prior to surgery.
The Bottom Line
Topical antibiotics can provide outstanding surface sterilization and therapeutic intracameral and intrastromal bactericidal concentrations. Improved protection with next generation topical fluoroquinolones against perioperative infections or conjunctivitis15 has already been documented in vitro and in animal models. Fluoroquinolones are firmly established as the drug class of choice for these indications, due to superlative spectrum and toxicity profiles when compared to other available topical agents.
Topical prophylactic antibiotic use, even though never unequivocally proven to prevent post-cataract endophthalmitis, has become an integral part of the perioperative regimen for most surgeons. Although this is not an established community standard for care, continuous pressure to provide this added protection comes from numerous fronts: colleagues, patients, pharmaceutical companies, risk-management underwriters, and a growing body of scientific evidence. Therefore, selection of the most appropriate and effective agent is central to providing the best possible care for our patients. This intense concern for better outcomes and fewer complications applies not only to cataract surgery, but to refractive surgery as well as patients suffering from bacterial conjunctivitis. Newer fluoroquinolone agents offer improved spectrum, better solubility, greater penetration and, thereby, superior efficacy.
The next generation of fluoroquinolones, regardless of numerical assignment, raises expectations even higher, ultimately, we hope, to the benefit of our patients. Clinical data directly comparing the next generation, levofloxacin, gatifloxacin and moxifloxacin, is sparse. As clinicians, we await unbiased in vivo human clinical data collection and purposeful scientific analysis. Until then, we can be pleased that continuous pharmaceutical advances have been made to counter the rising tide of endophthalmitis, avoid the tragedy of blinding infection after elective refractive surgery, and provide truly efficacious broad spectrum coverage for bacterial keratitis and contagious bacterial conjunctivitis.
Dr. Sheppard is an associate professor of Ophthalmology, Microbiology and Immunology at Eastern Virginia Medical School, director of the Uveitis Service & Ocular Microbiology Lab, and clinical director of the Lee Center for Ocular Pharmacology. Contact him at Docshep@hotmail.com.
1. Allen, HF, Margiaracine AB: Bacterial Endophthalmitis after Cataract Extraction: Incidence in 36,000 Consecutive Operations with Special Attention to Preoperative Topical Antibiotics. Arch Ophthalmol 1974;91:3-6.
2. Gills, JP; Filters and antibiotics in irrigating solutions for cataract surgery. J Cataract and Refract Surg 1991:17(3), 385.
3. Gimbel HV. Letter to the Editor, Ophthalmology 2000;107:1614-15.
4. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence based update. Ophthalmology 2002;109:13-24.
5. Jensen MK, Fiscella RG, Olsen RP. Comparison of Endophthalmitis Rates Over Four Years Associated with Topical Ofloxacin Vs. Ciprofloxacin: Moran Eye Center Retrospective Post-Cataract Endophthalmitis Study. ARVO 2002.
6. Nagaki Y. Bacterial endophthalmitis after small incision cataract surgery. JCRS 2003;29:20-26.
7. Hariprasad SM, Mieler WF, Holz ER: Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol 2003;121(3):345-50.
8 . Garcia-Saenz MC, Arias-Puente A, Fresnadillo-Martinez MJ, Carrasco-Font C. Human aqueous humor levels of oral ciprofloxacin, levofloxacin and gatifloxacin. J Cataract Refract Surg 2001 Dec;27(12):1969-74.
9. Ng EW, Samiy N, Ruoff KL, et al. Treatment of experimental Staphylococcus epidermidis endophthalmitis with oral trovafloxacin. Am J Ophthalmol 1998;126(2):278-87.
10. Bucci FA. An in vivo comparison of the ocular absorption of levofloxacin versus ciprofloxacin prior to phacoemulsification. ARVO:B589, 2002.
11. Levine, JM, Noecker RJ, Snyder RW, et al. Comparative Penetration of the Fluoroquinolone Antibiotics Gatifloxacin and Levofloxacin into the Rabbit Aqueous Humor after Topical Dosing. ASCRS Abstract B729, 2002.
12. Andriole VT, ed. The Quinolones. 3rd edition. London: Academic Press. 2000:477-491.
13. King DE, Malone R, Lilley SH. New classification system and update on the quinolone antibiotics. Am Fam Physician. 2002 May 1:16(9):2741-8.
14. Browne, FA, Clark C, Bozdogan B, et al. Single and multi-step resistance selection in Streptococcus pneumoniae comparing ceftriaxone with levofloxacin, gatifloxacin, and moxifloxacin. Int J Antimicrobial Agents 2002:93-99.
15. Graves A, Henry M, O'Brien TP, et al. In vitro susceptibilities of bacterial ocular isolates to fluoroquinolones. Cornea 2001,20(3):301-305.



