The race to create a working accommodative intraocular lens—one that can give patients something resembling the visual range most of us had in our youth—continues. In the June issue of Review we profiled three accommodating lenses in the pipeline (the Juvene, the Lumina and the FluidVision lens). This month we add two more to the list of aspiring contenders.
The Atia Vision Lens
The Atia Vision modular presbyopia-correcting intraocular lens (Atia Vision, Campbell, California) features a modular, two-part design. The back part of the device is a shape-changing “accommodating engine” that works using what the company calls a “hydraulic multiplier,” intended to mimic the mechanism used in natural accommodation. This part of the device maintains direct contact with the capsular bag to maximize energy transfer between the ciliary muscle and the optic. The front section of the device is an exchangeable optic that’s used to address the specific refractive needs of the individual patient. This part will be available in multiple powers and degrees of toricity, and should allow for future upgrades as refractive technology advances.
 |
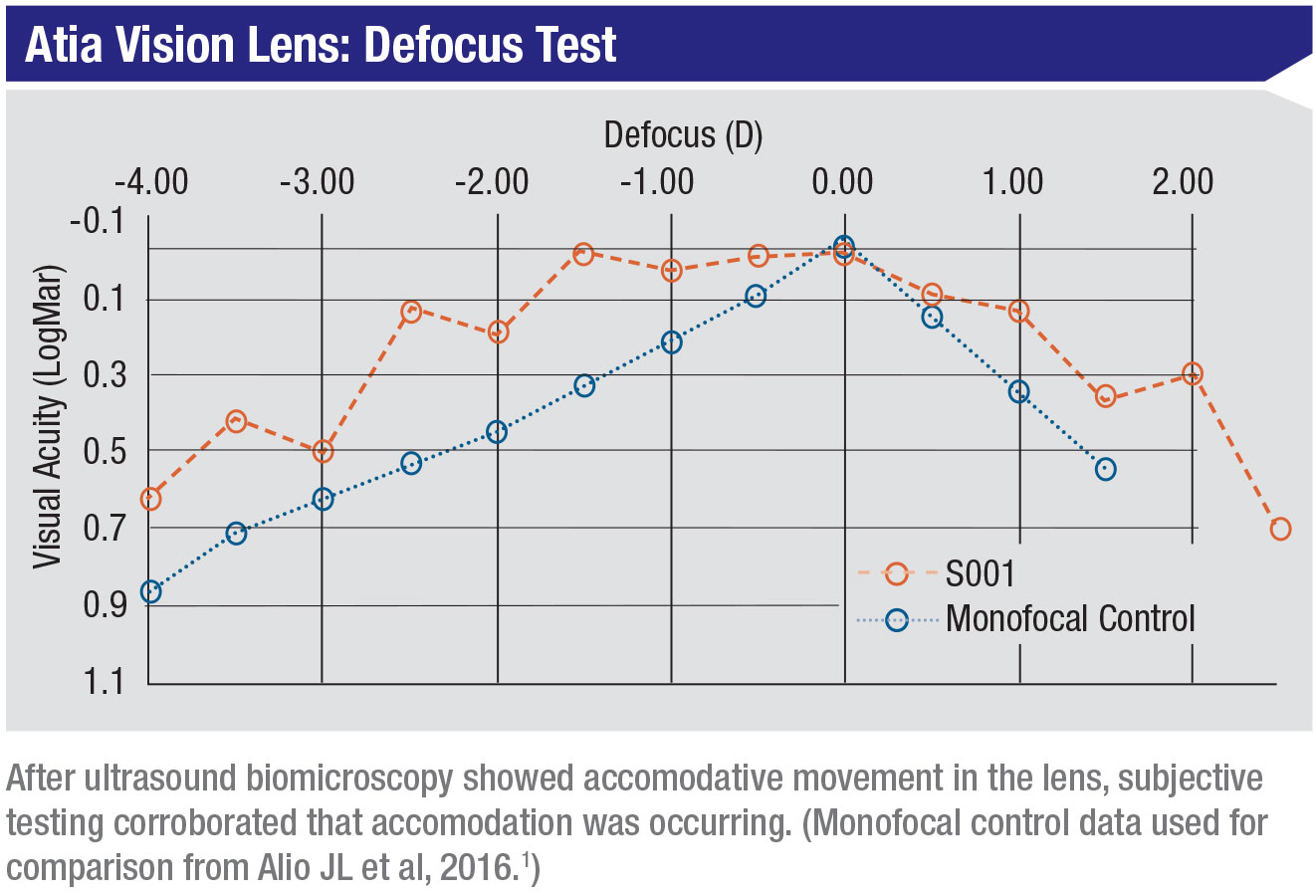
In preliminary (not-in-human) testing of the Atia Vision lens, both subjective and objective measurements confirm that accommodation is taking place inside the eye. No significant product-related adverse events have been seen to date.
P. Dee G. Stephenson, MD, FACS, president of the American Board of Eye Surgery, is familiar with the lens. “The goal of the Atia Vision lens design is to restore a full range of functional vision to patients,” she explains. “Previous accommodative lens designs have produced limited accommodative response, difficult surgical procedures and unpredictable refractive outcomes.
“The Atia features a modular design that should prevent visual disturbances and artifacts,” she continues. “The shape-changing engine maintains direct contact with the open capsular bag to allow efficient energy transfer from the ciliary muscle, while the front optic is designed for refractive predictability and astigmatism control. It’s easily exchangeable.
“Because of the modular design, implantation is a two-step procedure,” she adds. “Otherwise, the surgeon follows normal cataract surgical procedures. Currently, first-in-human studies are under way in Europe. The results of those studies will inform future modifications of the lens.”
For more information, visit atiavision.com/#company.
 |
The Opira Lens
Another accommodative IOL in the pipeline is the Opira AIOL, from ForSight Vision6 (Menlo Park, California). The Opira is a dynamic, shape-changing lens, designed for placement in the sulcus (haptic-fixated within the capsulorhexis). The company says this placement allows direct ciliary body engagement without zonular or capsular bag intermediaries. (The company acknowledges that placing a lens inside the capsular bag may seem more intuitive, but that differences in capsular bag dimensions, elasticity, and healing/fibrosis factors between patients are problematic.)
 |
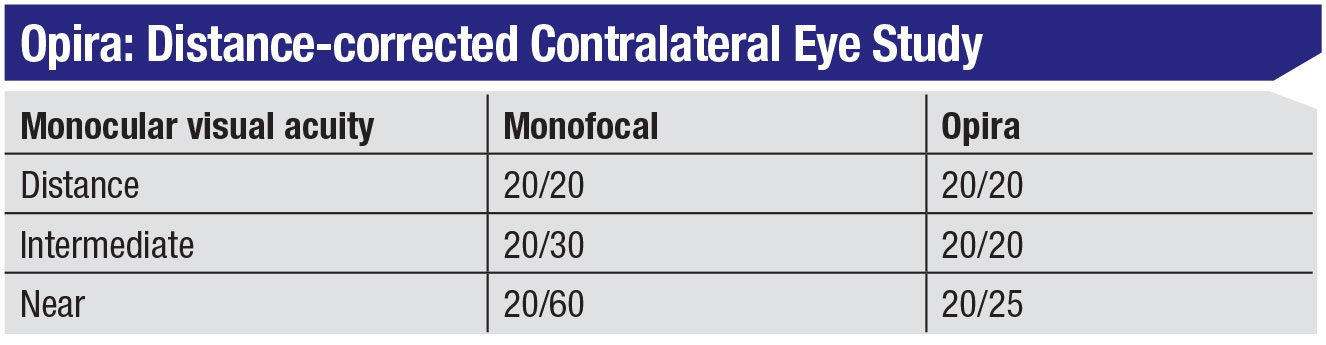
The anterior surface of the Opira lens is dynamic; a static posterior lens can be used to correct regular astigmatism or for postoperative refractive adjustment. The company says the lens is easy to implant, requiring less than three minutes to position. An early clinical study involving 16 patients with a nine-month follow-up compared contralateral eyes, one implanted with a monofocal, the other with an Opira lens. The study found that when the eyes were corrected for distance, both lenses were 20/20 at distance, but at near the monofocal was 20/60, while the Opira lens was 20/25.
“The primary characteristic that distinguishes the Opira AIOL from many others is that it employs direct ciliary body engagement,” explains Ayman Naseri,
MD, president and CMO at ForSight Vision6. (Dr. Naseri is also a professor of ophthalmology at the University of California San Francisco.) “It doesn’t rely on the elasticity of the capsular bag as an intermediary for dynamic shape change. Although the Opira lens is haptic-fixated within the capsule, direct engagement of the ciliary body allows robust accommodative function without relying on an important variable in pseudophakic accommodation—capsular bag elasticity and size differences between patients and over time.”
 |
Dr. Naseri says the data from the clinical studies conducted so far have been very promising. “They’ve demonstrated excellent safety and visual acuity, including monocular distance-corrected vision of 20/20 at intermediate and 20/25 at near,” he notes. “Also, the Opira defocus curves are flat at the top. That’s consistent with true accommodation.”
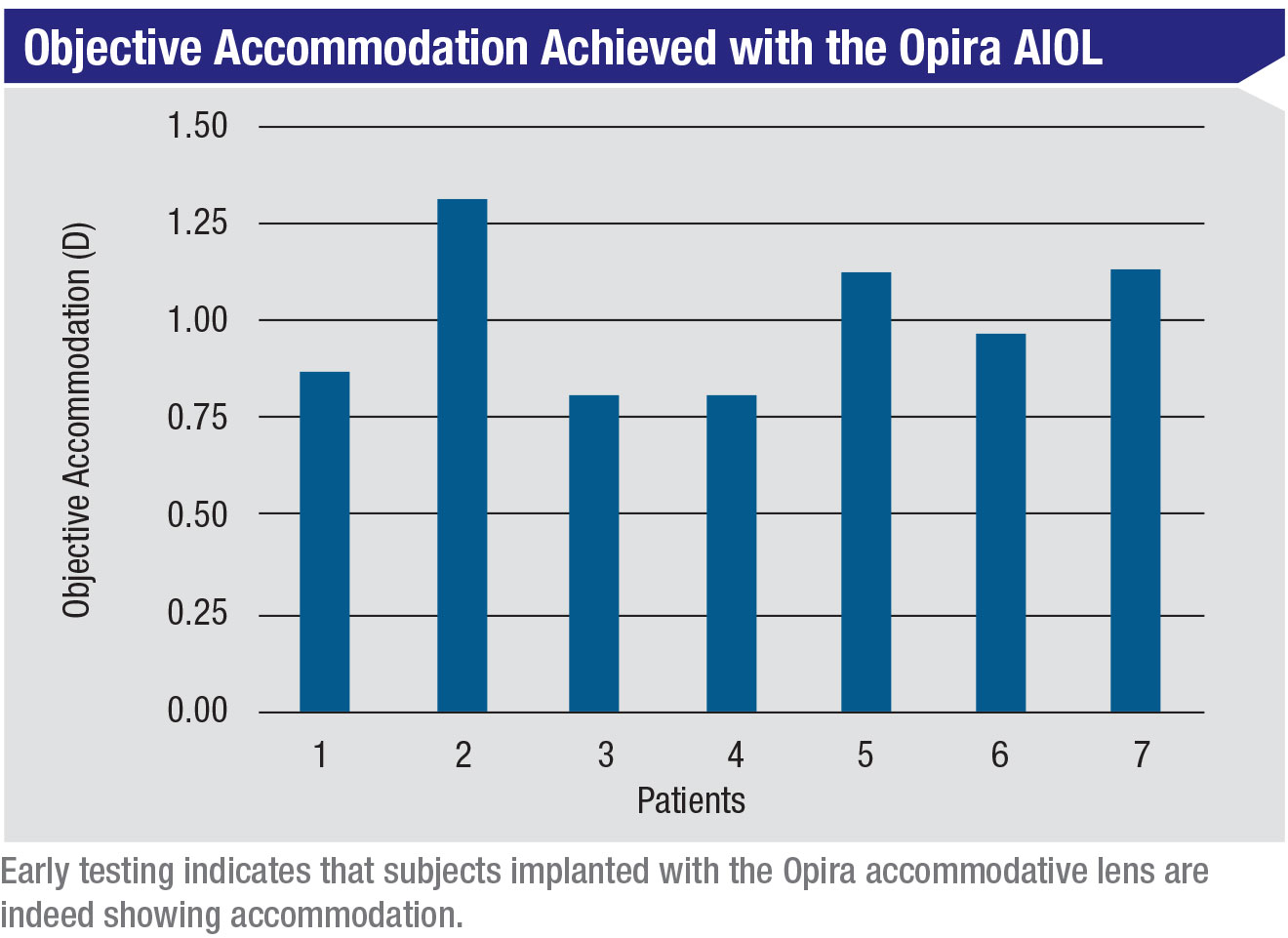 |
Dr. Naseri says the Opira is implanted using an injector via a temporal clear corneal incision. “The insertion is straightforward and intuitive,” he says. “It takes about one minute longer than the time required to insert a monofocal IOL.”
The company is currently recruiting new patients for a proof-of-concept trial to demonstrate 1 D of objective accommodation with the lens. “In terms of what’s next, we’ll continue fine-tuning certain aspects of the Opira lens as we conduct additional clinical studies,” Dr. Naseri says. “It’s availability in the U.S. marketplace will depend largely on the FDA regulatory approval process, which we’re preparing for now.” REVIEW
1. Alio JL, Simonov A, Plaza-Puche AB, et al. Visual outcomes and accommodative response of the Lumina accommodative intraocular lens. Am J Ophthalmol 2016;164:37-48.