Preop Workup is Critical
“I can have a long eye, a short eye or a post-LASIK eye, but the most important thing I do is give them all the same thorough preoperative workup,” emphasizes P. Dee Stephenson, MD, FACS, president of the American College of Eye Surgeons, associate professor at University of South Florida College of Medicine in Tampa and CEO/CFO of Stephenson Eye Associates. “I use the IOLMaster 700, the Cassini and the iTrace on every patient. I also do an OCT of the macula to make sure there is no pathology.”
Samir Sayegh, MD, PhD, FACS, of the Eye Center in Champaign, Ill., also considers a meticulous workup fundamental to a good refractive outcome. “We have a routine that applies to all eyes identified as being particularly exceptional,” he says. “We do a lot of repeat testing. We do partial coherence interferometry using the IOLMaster for axial length, and we do ultrasound. We do both every time, for every patient. For the measurement of the K value, we use at least three methods. Another thing we do is OCT of the retina for all patients. We also do pachymetry on all patients.”
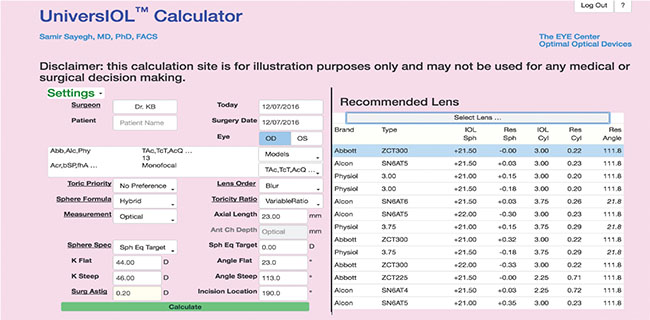 |
| The UniversIOL Calculator’s database includes a vast array of lenses. It also allows surgeons to enter spherical and toric data simultaneously and features multiple computation modes, including the Hybrid function, which runs multiple formulas and selects the best one. |
Dr. Sayegh says these measures help to reveal any pathology ahead of time. “Everybody having cataract surgery will get OCT, and they will get evaluation of the thickness of the cornea, so that if there is underlying Fuchs’ or anything that would be an issue at the time of surgery, we can take extra care with the eye. If there’s anything that would show up later, in the postop results, we also want to know ahead of time.”
“Anybody that comes into our office for an exam gets an aberrometry scan,” says Brock K. Bakewell, MD, FACS, partner at Fishkind, Bakewell & Maltzman Eye Care in Tucson, Ariz., and adjunct associate professor of ophthalmology at the University of Utah. “We do an OPD using the Nidek system for aberrometry. I can look at that and usually tell if they’ve had myopic or hyperopic LASIK just from looking at the aberrometer. You know how much spherical aberration patients have, and that dictates what lens you’re going to put in the eye if you’re going to try and balance that for the best optical outcome.
“The aberrometry is great, but you obviously also need a good examination at the slit lamp,” continues Dr. Bakewell, who adds that he’s had some patients forget to tell him about refractive surgery performed 15 or 20 years ago.
Stabilize the Ocular Surface
“Anybody who’s doing cataract surgery needs to be aware of corneal surface disease,” states Dr. Bakewell. “Dry eye can make your biometry measurements very inaccurate. If you get a lot of variability in your measurements, you should put your patients on drops and tune up the corneal surface before you take their final measurements prior to cataract surgery.” He adds that map-dot-fingerprint surface dystrophy (anterior basement membrane corneal dystrophy) and Salzmann’s nodules are two findings that must be addressed before doing final biometry. “If Salzmann’s nodules affect the central cornea at all, they should be scraped two to three months prior to doing your measurements for cataract surgery,” he says.
Dr. Sayegh adds, “Once you have a very good assessment of the state of the cornea with regard to astigmatism and any dryness, and you treat and stabilize it, then there’s no reason to measure differently than with other eyes. Make sure that you have a stable K for a consistent reading.”
Use Modern, Tested Formulas
Repeated measurements of a stable eye facilitate good IOL power calculations. So does the use of modern, yet tested formulas. “I look at all of the data I’ve collected and run calculations using multiple formulas,” says Dr. Stephenson of her next step after workup. She has a repertoire of formulas that she uses consistently. “Right now, I use SRK/T, Barrett, Koch and Haigis,” she says, adding that she notes she’s been using more Barrett and less SRK/T and Haigis lately.
“The third- and fourth-generation formulas are the ones we should be using now,” says Dr. Bakewell. “We used to run Holladay 1 and Holladay 2. Now our standards are pretty much the Barrett, the Olsen, and Warren Hill’s new formula, the radial basis function (RBF).” He says that he runs this trio of formulas on every patient. “If you’re trying to hit zero correction, using those formulas is going to get you within about half a diopter, plus or minus, of your target about 90 percent of the time,” Dr. Bakewell estimates.
Dr. Sayegh and colleagues have developed the web-based UniversIOL Calculator (2020eyecenter.com/iol-calculator/) to help make searching for the single best formula for a given eye a thing of the past. “Everybody and their brother or sister has a formula,” he quips. “But there are some that have stood the test of time. Some prove very effective, very consistently over large groups of eyes.” Dr. Sayegh singles out the Haigis-L formula as one such example. His calculator incorporates third- and fourth-generation formulas and allows the surgeon to enter spherical data and toric data at the same time without switching from one calculator to another. It also contains ranked data for every IOL manufactured worldwide.
The UniversIOL Calculator will guide the selection of correctly powered IOLs for any lens model, but the surgeon can override its recommendations. Users can also run one or multiple formulas simultaneously. “My calculator has a function called Hybrid, were it calculates all of them and it selects the one that is the best for that particular eye,” Dr. Sayegh explains.
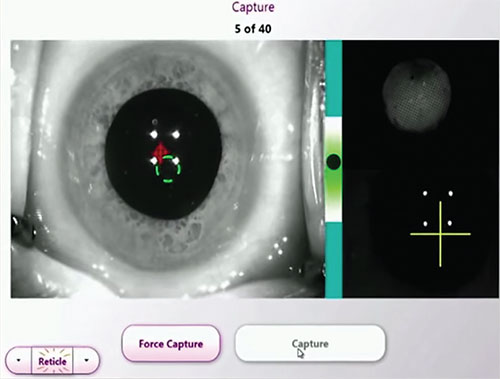 |
| Intraoperative aberrometry can confirm or refine IOL power estimates. |
He adds that the Hybrid function is important because formulas may demonstrate instability when applied to certain difficult eyes. “Certain combinations of Ks and axial lengths in the SRK/T, for example, are unstable and will not give you a result that you should rely on,” he explains. “So if the eye we are looking at is in that region, we exclude the SRK/T from being calculated. You can override the system and say you want to use the SRK/T anyway, but our Hybrid algorithm always chooses one formula that’s consistent with all the published literature and is established to be the best in that parameter set.”
Dr. Sayegh reports encouraging refractive outcomes. “People at -15 D, -16 D come very comfortably within ±0.5 D of target,” he says. “Very often we get within ±0.25 D. The results are really very good. “
Post-refractive Surgery Eyes
Patients with prior refractive procedures may be expecting the same dramatic visual improvement after cataract surgery with IOL implantation that they enjoyed after LASIK, PRK or RK. “Prior refractive surgery patients have very high expectations,” says Dr. Bakewell. “You always have to tell them that even with all the formulas we run, everything is a best guesstimate, and they still might come out a diopter wrong. If they do, I tell them I won’t make them live with it. I’ll offer them the option of exchanging the lens, for example.”
Dr. Stephenson finds both the ASCRS calculator and the IOLMaster helpful in these eyes. “Optimizations for different post-refractive eyes are done for you using the IOLMaster after you plug in the data you have,” she notes.
“Hopefully, anybody who has had RK has already had their cataract out,” says Dr. Sayegh, adding that he hopes the procedure is now “a historical aberration that we don’t have to encounter often.” Faced with such an eye, Dr. Sayegh says he would refract it with a contact lens and then target for slight myopia. “If you implant something and they’re still -3 D or -2 D and they don’t like it, you wouldn’t want to mess with their cornea. What you can do is implant a piggyback IOL. I think we’ll get a few more of them in the United States in the next few years.”
“We have an Orbscan for topography, and we do total axial power measurements on anybody who’s had myopic PRK or LASIK or RK,” says Dr. Bakewell. “Averaging the four central keratometry readings from the total axial power measurements gives an average K that we run in the Barrett formula. Since this average K is usually slightly flatter than the true K readings, one must choose an IOL power that shoots slightly on the hyperopic side, approximately 0.25 to 0.5 diopters. The ASCRS calculator for post-refractive surgery is also one of the best things to use, but still requires some interpolation due to the range of suggested IOL powers.”
That is thought to be in part because myopic and hyperopic ablation procedures flatten or steepen the cornea, respectively, throwing off assumptions about corneal power in IOL formulas developed for surgically virgin eyes. Also, after myopic LASIK, IOL formulas relying on the relationship between anterior chamber depth and the steepness of the cornea to estimate the effective lens position can erroneously predict an artificially shallow lens position, leading surgeons to select an underpowered IOL, which plays a role in hyperopic surprise. After hyperopic LASIK, the surgically steepened cornea can lead to the opposite error: artificially deeper effective lens placement estimate and an overpowered IOL selection, a causative factor in myopic outcomes.2
“If they’ve had myopic LASIK, I’d rather leave patients slightly myopic versus hyperopic. Patients hate hyperopia. The other thing is that it’s easier to correct myopia with PRK than hyperopia,” says Dr. Bakewell.
Of prior hyperopic LASIK patients, he says, “Their Ks are a tiny bit steeper. They’re read by the Lenstar or IOLMaster as a little bit flatter than they really are. For a patient with previous hyperopic LASIK, you’re going to pick a lens that suggests a slight hyperopic result.”
If you do end up with a small refractive surprise and an unhappy patient, resist intervening too soon, says Dr. Bakewell. “If you do a surgery and it comes out just 0.5 D or 0.75 D on the farsighted side, I would let the lens reside in the eye for approximately three months, because the refraction can change in the right direction. I recently had a patient who was hyperopic after myopic LASIK. She was +0.75 D after her cataract and IOL surgery. This lady was very unhappy, and wanted me to do something right away. I said, ‘No. We need to wait,’ and she ended up being -0.5 D three months later. So you don’t want to be too quick to do a PRK or an IOL exchange. You really want to wait those three months for the lens to settle in and see if it changes position in the capsular bag with healing.”
Short Axial Length
In eyes with short axial length the biggest challenge to a good refractive outcome is that the actual implant position may be more anterior in the eye than the estimated effective lens position indicates, causing calculation of an overpowered lens and a myopic surprise.
Dr. Sayegh believes that Hoffer Q is a good choice for IOL calculation in short eyes, based on consistent evidence in the literature. “There’s not one, but dozens of papers, and each of those papers has 100, 200 or maybe thousands of eyes, so there’s some consistency there, where you know that this has been tested again and again.”
Dr. Bakewell says he tends to gravitate towards the Olsen formula for short axial lengths. “I used to use the Hoffer formula, but that’s somewhat outdated. Holladay 2 is not bad, but I think the Olsen is probably best for short eyes.”
Long Axial Length
IOL calculations in eyes with long axial length can lead to selection of an underpowered lens and hyperopic outcomes for multiple reasons: difficulty in obtaining an accurate measure of the axial length; an increase in the prediction error in formulae as axial length increases; inaccurate ELP; and IOL constants not suited to the long eye.3 “If the eye is really long,” says Dr. Bakewell, “I won’t pick a lens that is projected to give me a zero result. I’m going to shoot for a lens that’s got maybe -0.5 D, -0.75 D or even -1 D. I’d rather patients come out a tiny bit myopic than move them into farsightedness.”
Dr. Sayegh considers the Wang-Koch modification optimal for long eyes. “If you have very long eyes, you can use the Holladay 1 or the SRK/T, for example, but for each of these formulas, they suggest you make a different corrective modification: You use not the true axial length, but the modified axial length, and they give you a formula to modify it.” There are discrete Wang-Koch modifications to find the optimized axial length using the Haigis and Hoffer Q formulas, as well. Surgeons can currently enter Wang-Koch modifications directly into the UniversIOL calculator, but Dr. Sayegh is considering adding a mini-calculator to the Hybrid feature that would figure out the Wang-Koch modification using the true axial length, and then feed the converted AL into the final IOL calculations.
Staphyloma
Staphylomatous eyes make measuring the AL challenging. “The staphyloma throws you off as to where to fovea is,” says Dr. Sayegh. “Your main problem is not which formula to use: The main problem is getting the correct axial length. We use partial coherence interferometry; we use ultrasound, doing multiple measurements with different devices, and we do a B scan. That will give you not just the length, but also the whole shape of the back of the eye. How the signal is bouncing can help you identify where the staphyloma is and get you a much more reliable reading. You want the distance between the cornea and the fovea. You also do OCT. With all of that information you determine what the true axial length is. You don’t have to use any magic formula for staphyloma.”
Dr. Bakewell emphasizes that being even a tiny bit lateral or nasal to the center of the staphyloma while trying to measure to the fovea can result in big refractive errors. “Being off one millimeter in the axial length translates to being off three diopters in lens implant power,” he emphasizes. He runs the Barrett, Olsen, and Hill formulas for these eyes using an AL measurement to the fovea, and he fudges towards myopia. “Your formulas sometimes underestimate the power of the lens to put into these folks,” he says. “Even if I’m trying to achieve plano, I’m shooting for -0.75 D or a -1 D, because frequently you’re off a little bit in that direction.”
Keratoconus and PK
Dr. Bakewell generally doesn’t target plano in keratoconic eyes. “If the patient is a successful contact lens wearer with gas-permeable lenses, and is planning on wearing contacts after cataract surgery, I usually shoot to leave those patients on the myopic side, maybe -2.5 D to -3 D. That lets them see well enough to put their contacts in. If their keratoconus is significant, they are always going to need a contact in order to see their best. There’s no reason to shoot for plano.”
If the keratoconus is severe enough, and the cataract is not too debilitating, Dr. Bakewell will do a corneal transplant procedure first, then wait approximately nine months to a year for most of the sutures to be out before doing cataract surgery. “In terms of calculations you just use your regular formulas, but many times they have very irregular astigmatism, so they’re difficult eyes,” he says. “In terms of cataract surgery, keratoconics have very steep corneas, but if you do a corneal transplant, you’re replacing their steep cornea with a flatter one. If you’re taking power away from their eye, then the lens implant is going to have to be stronger, assuming you’ve already flattened their cornea. If you’re doing cataract surgery prior to a corneal transplant, then you’ll have to put a steeper-powered lens in than what your measurements call for, because your keratometry measurements might be four, six or eight diopters flatter after corneal transplantation. It’s better to do the corneal transplant first if patients really need it. Your measurements are going to be much more accurate.”
Dr. Sayegh does not believe that there is an all-purpose IOL formula for keratoconic/PK eyes, either. “What matters is an effective reading of the K. The problem is that their Ks are essentially variable. You have to stabilize the surface of the cornea and then bring them back for measurements,” he says. “Post corneal transplant, you want to know what the potential vision was at the time of the corneal transplant, before they developed the cataract. Sometimes, the distortion at the level of the cornea is what really limits their vision, not the cataract. These patients can do well with toric IOLs.”
Dense Cataracts
Dr. Sayegh reports that he sees a sizeable subset of patients with dense cataracts both in the United States and abroad because of their difficulties accessing health care at earlier stages. “For the advanced cataract, even with the new IOLMaster and some of the new partial coherence interferometry systems that penetrate more dense cataracts, you sometimes have to go to ultrasound,” he says. “Ultrasound does it every time: It always gets results—not always as reproducible, but the differences are minimal—especially for patients who come to you with very poor vision in the first place.”
Dr. Stephenson says the IOLMaster 700 makes good axial length measurements feasible in most advanced cataract cases. She also suggests measuring an unaffected fellow eye to help zero in on axial length, provided that eye has a stable refraction.
Intraoperative Aberrometry
For patients with dense cataracts—or any cataract patients—intraoperative aberrometry can help surgeons corroborate or fine-tune IOL power choices in challenging eyes. Research comparing Optiwave Refractive Analysis (ORA) with three preop methods of IOL calculation (surgeon’s best choice; the Haigis- L, and the Shammas) in 246 eyes of 215 patients with a history of myopic LASIK or PRK demonstrated that eyes refracted intraoperatively with ORA had the lowest median absolute error at 0.35 D, and 94 percent of the ORA eyes were within ±1 D of the device’s predicted outcome.4
Tal Raviv, MD, FACS, associate clinical professor of ophthalmology at New York Eye & Ear Infirmary of Mount Sinai, and founder and medical director of the Eye Center of New York, describes intraoperative aberrometry as “invaluable for tough biometry cases such as very long axial length, mild to moderate keratoconus, and post-refractive eyes.
“In 2016 we have some very good ‘no-history’ IOL calculation methods available on the ASCRS calculator,” Dr. Raviv continues. “However, using ORA has been shown to be more accurate in some studies. Furthermore, it is not uncommon for post-LASIK eyes to require a toric IOL, and no preoperative measurement can perfectly measure the true anterior and posterior astigmatism of these eyes. ORA allows the surgeon to neutralize the refractive astigmatism with high accuracy.” He adds that surgical approach and sedation level are important considerations with ORA, since the device incorporates patient fixation into measurements. “During the Verifeye aphakic reading,” he explains, “one can see the cylinder measurements jump significantly with very slight movements of the eye.” Patient participation will yield more accurate results.
Dr. Stephenson goes in with her preop calculations prepared, but will move to what ORA recommends in the OR if there is a discrepancy. “The ORA can reference some 550,000 cases, and I’ll err on the side of what it tells me intraoperatively,” she says.
As IOL implantation inches closer to the long-term goal of emmetropia, surgeons implant challenging eyes with a more immediate goal: patient satisfaction and well-being. “You can plan all you want,” says Dr. Stephenson, “but sometimes there are surprises, and you have to look at the gestalt of the situation and make a decision that you think will be best for the patient.” REVIEW
Dr. Stephenson is a member of the speakers’ bureau for Cassini and ORA/Alcon. Dr. Sayegh reports no financial disclosures. Dr. Bakewell is a consultant for AMO. Dr. Raviv is a consultant for AMO.
1.Apple DJ, Sims J. Harold Ridley and the invention of the intraocular lens. Surv Ophthalmol 1996;40:4:279-292.
2.Patel RH, Karp CL, Yoo SH, Amescua G, Galor A. Cataract surgery after refractive surgery. Int Ophthalmol Clin 2016;56:2:171-182.
3. Zhang Y, Liang XY, Liu S, et al. Accuracy of intraocular lens power calculation formulas for highly myopic eyes. Journal Ophthalmol 2016(2016):Article ID 11917268, 7 pages. http://x.doi.org.10.1155/2016/1917268. Accessed December 9, 2016.
4.Ianchulev T, Hoffer K, Yoo SH, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology 2014;121:1:56-60.



