In late May, the Ophthalmic Devices Panel of the Food and Drug Administration unanimously recommended approval of the first accommodating intraocular lens, the CrystaLens from C&C Vision (Aliso Viejo, Calif.)
The Crystalens is a modified plate haptic silicone lens with a 4.5 mm biconvex optic diameter and an overall length of 11.5 mm. It has a hinged haptic adjacent to the optic and polyimide loops. The hinges are designed to minimize resistance and facilitate movement of optic.
 |
The clinical trials on which the approval was based included 324 patients, 497 eyes, with a mean age of 69.7 years and a range 47.7 to 88.4 years. Inclusion criteria called for patients scheduled for cataract surgery by phacoemulsification, with a potential for best corrected visual acuity 20/32 or better in each eye, corneal cylinder of 1.0 D or less, and one-year follow-up. Phase 1 was limited to monocular implant, while Phase 2 permitted bilateral implantation.
Michael Breen, OD, director of clinical outcomes for C&C, says that bringing the first accommodating IOL before the FDA for approval was new territory for the company and the panel. "Traditionally, in the IOL category what the panel had been interested in was best corrected distance vision," says Dr. Breen. "Probably the most definitive data that we took was measuring the near vision through the distance correction, and we did that to demonstrate the accommodative abilities of the lens by removing some of the confounding factors that may contribute to near vision, such as myopia and astigmatism.
"We looked at the equivalents of 20/20, 20/25, 20/32 and 20/40, where 20/25 essentially equates to J1 vision and 20/40 to J3. In the findings that we submitted, we found that 100 percent of the subjects, implanted bilaterally could achieve near vision measured through the distance correction of 20/40 or better." Most newspaper and magazine print, says Dr. Breen, is larger than the type that was used in testing.
The lens is thought to achieve accommodation through ciliary muscle contraction and relaxation that results in the redistribution of muscle mass. This leads to a change in pressure of the vitreous cavity and in the anterior chamber. As the optic locates against the vitreous face, these pressure changes move the optic forward and backwards.
The amount of lens movement was tested by several means. "In testing such as dynamic retinoscopy and in measuring the patient's near point of accommodation, we found a good range of accommodative amplitudes, ranging from 2.5 D up to more than 6 D," says Dr. Breen. "Our findings on dynamic retinoscopy, which is probably a more sensitive tool for assessing that, ranged from 2.25 D to more than 4 D of accommodation. So we found varying amounts but what we submitted to the FDA allowed them to be comfortable with giving us the designation of accommodating IOL."
Mary Ann Tingley, marketing director for C&C, points out the lens's position in the eye overcomes the potential glare and halos that would be an expected with the smaller, 4.5-mm optic of the CrystaLens. "The A constant on the lens is 119.24," says Ms. Tingley, "so it sits very far back in the eye. Because of that and its excellent centration, glare and halos are minimized, and the clinical trials supported that. A doctor may wonder, 'How can that be when I get glare and halos with a 6 mm optic?' The answer appears to be that the lens is sitting so far back and so close to the nodal point that the light is focused."
Lynne Archer, director of clinical research, directed a lifestyle survey of the study participants. Though the study was based on patient recall and variable parameters (e.g., the study could not assess the size of type or the contrast of a newspaper a respondent may have read), "what we came away with was that more than half of the people could sit down and read a prescription bottle of 20/25," says Ms. Archer. "Nearly everybody could perform all functions at all times except for maybe that very tiny writing, maybe in a restaurant under dim lighting."
There are no contraindications to using the lens other than the standard ones that apply to bag-fixated IOLs generally, such as issues with zonular support or pseudoexfoliation. Only two lenses in the trials required explantation, one for an incorrect power determined by the manufacturer, and the other due to capsulorhexis that was made too large. C&C Vision is now finalizing labeling terms with the agency, which generally accepts the recommendations of the panel, and formal approval may come by the fall of this year or early in 2004.
WIO Honors Bruce Wong
Women in Ophthalmology presented its Spring Lectureship Award to Bruce Wong, MD, executive director of EyeCare America, in recognition of the American Academy of Ophthalmology's commitment to education and public service. Dr. Wong accepted the award at the WIO Spring Lecture held April 13 at the ASCRS meeting.
Advanced Medical Optics made the award possible by providing an unrestricted grant that will support two speakers per year, one at each of WIO's spring (ASCRS) and fall (AAO) meetings.
Of AMO's support, President and CEO James V. Mazzo said, "Fostering support and networking opportunities among female ophthalmologists will prove to advance the field overall." Marguerite McDonald, MD, called AMO's support of Women in Ophthalmology a "vote of confidence," saying, "The grant from AMO will allow WIO to have first-rate speakers at our meetings, thereby helping us to strengthen and grow the organization—the only one of its kind."
Founded in 1978, Women in Ophthalmology is dedicated to enhancing the professional environment, encouraging diversity, impartiality and economic parity, and to cultivating new opportunities for leadership, education and public service.
FDA Approves CIBA's Focus for Therapeutic Use
The FDA has approved CIBA Vision's Focus Night & Day lenses to be used as bandages to protect the cornea and relieve corneal pain following surgery and in the treatment of acute or chronic ocular pathologies.
CIBA reports that the disposable soft lenses supply up to six times the oxygen to the eye of ordinary contact lenses and can be worn for up to 30 continuous nights, making them ideal for therapeutic use since wearers won't have to repeatedly remove and insert them, and they're disposable. The lenses are made from a new silicone hydrogel material and have a unique biocompatible surface that helps minimize protein and lipid-deposit buildup over time.
The lenses are available in power ranges from +6 D to -10.00 D in two base curves. For more information, visit cibavision.com.
Minocycline May Benefit Blepharitis Patients
Minocycline, an antibiotic in the tetracycline class, may offer a new weapon against lid disease, according to research at the University of Texas Southwestern Medical Center. Investigators there studied the effect of oral minocycline on meibomian-gland lipid composition in chronic blepharitis patients. Their discoveries on the drug's mechanism of action may lead to a better understanding of the disease's pathology and the biochemical changes that result in the improvement of symptoms. The study appeared in the April issue of Experimental Eye Research.
 |
In the study, patients had acne rosacea with meibomianitis (AR-MKC), acne rosacea without ocular disease (AR) or seborrheic blepharitis (SBBL). Each group was given a daily 50-mg dose of oral minocycline for two weeks, followed by a daily 100-mg dose for a total of three months. Participants were followed for an additional three months with no treatment.
Results showed that oral minocycline reduced diglycerides and free fatty acids more significantly in the AR-MKC group compared to the AR and SBBL groups. And the therapeutic effects lasted three months after treatment was discontinued, suggesting that the drug has a more lasting effect on ocular microflora and may be most effective if the treatment extended past three months.
The significance of the research, says study author James P. McCulley, MD, is that "minocycline decreases lipid degradation and returns the secretions to their normal composition. The prior assumption was that minocycline inhibited enzymes but had no antimicrobial effect, and that the benefit only lasted while taking the drug. We confirmed the enzyme-inhibition effect. We unexpectedly found out that it has effective antimicrobial properties on the ocular surface and lid flora, and we learned that its benefits continue after cessation of a short course of therapy."
Christopher J. Rapuano, MD, co-director of refractive surgery and attending surgeon at Wills Eye Hospital in Philadelphia, says, "What's interesting about the study is how minocycline works and that it has lasting effects. Meibomianitis is a chronic condition that's quite symptomatic for many patients, and this is evidence that there's good treatment for it. The question is, do the effects last beyond three months? And how does minocycline compare with doxycycline and tetracycline?"
While the study raises questions, it answer others. "Chronic [posterior] blepharitis is a common reason why people come in to our offices," says William Trattler, MD, an ophthalmologist at the Center for Excellence in Eye Care in Miami. "We want to know why do some people have poor tear-film quality. What is biochemically wrong with their tears and, in particular, their oil glands? The answers to two of these questions are found in the study. If we know that we need to kill the bacteria and improve the oils secreted by the oil glands, we can prescribe the best medications and treatments like minocycline, warm compresses for the eyelids and maybe suggest dietary changes to improve dry-eye symptoms. Before this study, we didn't know how minocycline worked."
Animal Model Illuminates AION
Researchers at the University of Maryland School of Medicine have developed a model of anterior ischemic optic neuropathy in the rat that they say will help physicians and companies develop better treatments in the future.
The model, developed by associate professor Steven Bernstein, MD, PhD, was designed to mimic the non-arteritic form of AION. Up until now, the only model of AION available was of the arteritic form, and involved blocking the posterior ciliary arteries in monkeys, producing a stroke of the outer retina and the area supplying the optic nerve. The challenge in developing a non-arteritic model has always been causing a stroke in the blood supply to the optic nerve without damaging the retinal supply.
"The non-arteritic form involves only the optic-nerve capillaries, and is the most common cause of optic-nerve related sudden vision loss, usually occurring in individuals with diabetes, hypertension or small disks," Dr. Bernstein explains.
In the model, a researcher first injects a dye into a rat, than uses a proprietary photoembolic method to stroke the optic nerve capillaries while allowing normal blood flow to the retina. The researchers use a special fundus contact lens to directly visualize the rat's optic nerve and invasive surgery isn't necessary.
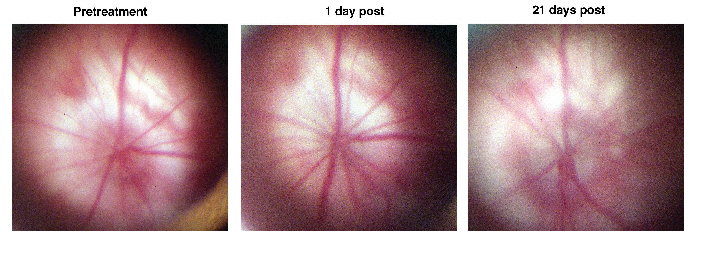 |
"It helps show us what changes occur in the eye after the stroke," says Dr. Bernstein. "And we now see that changes occur within a single day. So, even though you haven't produced a stroke directly within the retinal tissue, the retinal ganglion cells respond almost immediately. Before this, no one really knew how long it took the ganglion cells to undergo the programming involved with the decision to die. This new study with this model hints that the critical period for administering effective treatment to an AION victim may be very short." He says they've also learned that retinal neurons apart from retinal ganglion cells may also begin to react to optic nerve stroke within three days and show early signs of scarring.
Dr. Bernstein says that now that he has a model, he and his colleagues can begin testing individual agents to see how effective they are in treating non-arteritic AION. "We've isolated a number of genes that are targets for neuroprotective strategies," he says. "We can not only reproducibly generate an optic nerve stroke of any severity, but can rapidly quantify the precise amount of damage quickly, and determine what drugs are potentially effective in treating AION and which aren't."
New Formulation Acular Approved
The FDA also approved Allergan's Acular LS (ketorolac tromethamine ophthalmic solution) 0.4% for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
Acular LS, a new, optimized formulation of ketorolac tromethamine, joins a family of products used for a range of other conditions including post-surgical inflammation, pain, photophobia and ocular itch associated with seasonal allergies. Acular LS is expected to launch later this year.
Allergan says that in clinical studies, Acular LS significantly reduced pain, burning and stinging following corneal refractive surgery. The most frequently reported adverse reactions for Acular LS ophthalmic solution, occurring in approximately 1 to 5 percent of the overall study population, were ocular redness, swelling and pain, corneal infiltrates and headache.



