Today, ophthalmologists have a number of alternatives when it comes to addressing corneal disease with a transplant, ranging from the traditional full-thickness penetrating keratoplasty to partial transplants such as deep anterior lamellar keratoplasty and Descemet’s stripping endothelial keratoplasty, to an artificial cornea such as the Boston keratoprosthesis (aka KPro). Unfortunately, glaucoma often follows a corneal transplant.
The prevalence of glaucoma after a corneal transplant varies depend-ing on the type of transplant that was done, as well as other factors such as the presence or absence of pathology in the anterior segment. For example, greater prevalence and severity of glaucoma are seen in PK and KPro transplants because of the anatomic changes associated with the surgery. These changes lead to an increased likelihood of postoperative pathology in the anterior chamber angle, leading to more frequent ocular hypertension or glaucoma. If associated preoperative corneal or anterior segment pathologies remain after the surgery, the likelihood of glaucoma is even greater.
On the other hand, the prevalence of glaucoma following transplants such as DALK and DSEK is primarily affected by the nature of the pathology that led to the transplant. For exam-ple, the incidence of glaucoma fol-lowing DSEK is lowest when the transplant was necessitated by Fuchs’ dystrophy, because there’s not too much pathology in the anterior chamber. The same is true for PK necessitated by keratoconus. These eyes have better outcomes in terms of postoperative glaucoma.
Of course, one factor that’s especial-ly likely to increase the prevalence of glaucoma following corneal sur-gery is that the patient may need to use steroid drops to help with the postoperative healing. It’s no secret that patients can have a steroid response—ocular hypertension—which can lead to glaucoma over the long term. I actually consider any pa-tient with a corneal transplant to be a glaucoma suspect—especially those on steroids.
The reality that these patients need to be monitored for ocular hy-
pertension and glaucoma raises an interesting question: Does the presence of corneal pathology and/or having undergone a corneal transplant impact our ability to accurately measure intraocular pres-sure? Here, I’d like to discuss this question in two parts: First, we’ll talk about how corneal morphology and surgery can affect IOP measurement. Then, I’ll review the commonly used tonometers and discuss how best to use them in patients with corneal transplants and pathologies.
When Does Thickness Matter?
When measuring IOP, usually the first thing we consider is the corneal thickness. Thanks to the Ocular Hy-pertension Treatment Study, we know that patients with healthy, thin corneas (less than 550 µm) have falsely low pressure readings when measured with Goldmann tonometry. Likewise, patients with thicker-than-average corneas have falsely high pressure readings. It turns out, however, that once pathology and/or surgery become involved, that relationship becomes far less consistent.
Of course, both pathology and surgery can affect corneal thickness. For example, both keratoconus and LASIK surgery will leave the eye with a thinner cornea, and either circumstance can result in falsely low IOP measurements. However, the re-verse isn’t always true. If the cornea has been made thicker, whether by pathology or surgery, the result may not always be a falsely high reading, as you would expect with a thick, healthy cornea. For example, if corneal edema is present, the pressure reading taken through the thicker cornea may be falsely low rather than high.
In 2008 our group published a study involving DSEK that compared pressure readings using different tonometers.1 We checked the pres-sure in 50 eyes of 38 patients who’d undergone successful DSEK (mean-ing they didn’t have corneal edema), using a Goldmann tonometer, a Pneumatonometer and a Dynamic Contour Tonometer. We found that the readings were highly positively correlated. When one went up, the other went up, and vice versa. However, they were not interchangeable; the measurements were different from one device to the next.
Because DSEK leaves the cornea thicker as a result of adding tissue to the posterior surface, we wondered whether the increased thickness would cause falsely high pressure readings. Although the post-DSEK corneas were thicker, the Goldmann tonometer pressure readings were not falsely high. At the practical level, this means that if you get a high IOP reading in a post-DSEK eye using Goldmann tonometry, you should have a high index of suspicion that the IOP really is elevated.
The point is that once the cornea has been altered by pathology or surgery, the OHTS findings relating to corneal thickness and IOP measurement may not apply. It appears that if the cornea is thinner for either reason, you may get a falsely low reading, as you would with a healthy thin cornea. But if pathology such as edema or a surgical procedure has left the cornea thicker, the pressure reading (by Goldmann tonometry, at least) may actually be falsely low rather than high.
 |
Structure and Biomechanics
The structural and biomechanical properties of the cornea—including ectasia, hydration, scarring, curvature, astigmatism and hysteresis (a corneal property that isn’t fully understood)—can all impact the accuracy of our pressure readings. Scar tissue, for ex-
ample, can cause a falsely high read-ing. The complexity and variability of some of these factors—as well as how we take the measurement and which instrument we use—make it challenging to predict whether or not they’ll alter our IOP measurements.
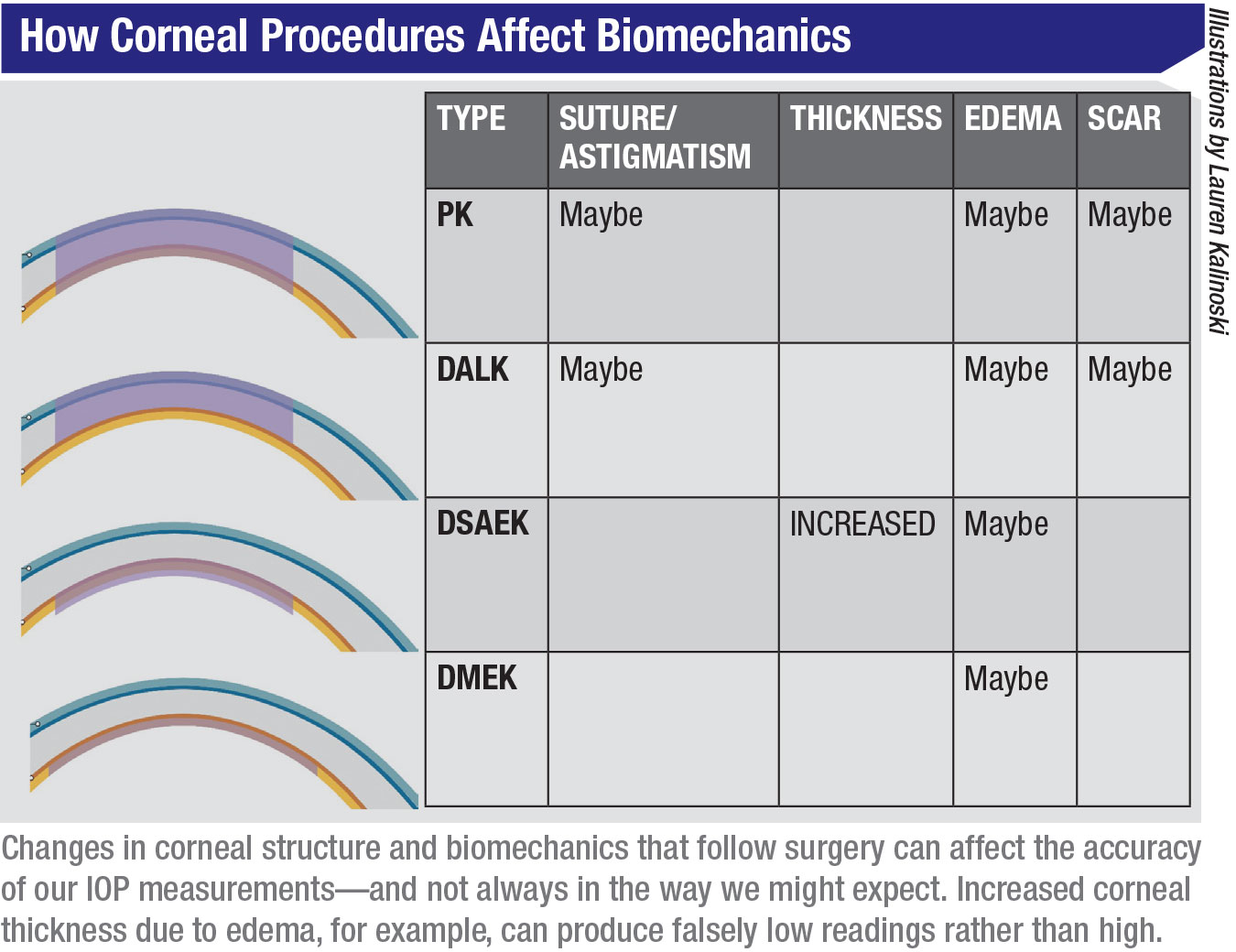
The table above summarizes how different surgeries can affect the biomechanics of the cornea, in turn potentially altering the accuracy or our pressure readings. For example, all corneal transplants are prone to causing edema, which may affect our IOP readings. Likewise, sutures and corneal scars, more commonly seen after PK and DALK, can influence the IOP reading. In addition, these surgeries may be altering the biomechanical properties of the cornea in other ways we don’t yet understand.
The bottom line is that when any corneal parameters have been altered by pathology or surgery, we can’t assume that our IOP measurements will be unaffected—or that we can always predict in which direction the readings will be altered.
Tonometer Technology
Clearly, when an eye has undergone corneal surgery or has pathology, the type of technology and specific instrument we use to measure IOP will affect the accuracy and consistency of the measurement. Fortunately, today we have several options for making that measurement. (Tactile estimation of IOP can also be useful, especially in patients who’ve had keratoprosthesis. Although this approach isn’t helpful for measuring subtle abnormalities of IOP, it is useful for identifying patients with very high or low IOPs.2,3)
Most tonometer technology to-day is based on one of three principles: applanation (flattening); contour matching; or rebound measurement. Most tonometers used in the clinic fall into the first category—they use corneal flattening or applanation. These include: the Goldmann tonometer, the Pneumatonometer, the Tonopen and the Ocular Response Analyzer, which uses bidirectional non-contact applanation.
A second technology that can be used to measure IOP is contour matching, used in the Dynamic Contour Tonometer. The interface resembles a little cup. Once you match the curvature of the cup to the curvature of the cornea, the device reads the pressure inside the eye. This technology appears to be less affected by corneal properties; as a result, it may give a reading that’s closer to the actual pressure inside the eye (in both healthy and post-surgical eyes).
The last category is rebound tonometry. Devices using this technology have a small tip that pushes gently but rapidly against the cornea. The device detects the deceleration of the tip—how quickly it stops. Devices in this category include the Icare tonometer, which measures through the cornea, and the Diaton device, which measures through the eyelid. (The latter is not commonly used in the clinic at present.)
Next, let’s consider the pros and cons of the most common tonometers.
Goldmann Tonometer
The Goldmann tonometer is still the gold standard for use in the clinic; it measures IOP using the Imbert-Fick principle. (This principle states that the pressure inside a thin-walled sphere can be determined by measuring the force necessary to flatten the surface, divided by the area of flattening.) It has an applanation diameter of 3.06 mm.
The biggest advantage of the Goldmann tonometer is that it’s readily available in the clinic today. However, it’s an imperfect technology. First of all, the device was calibrated to a corneal thickness of 520 µm and it assumes that the cornea is a perfect sphere. Second, its measurements can be affected by changes in corneal thickness, curvature, edema and surface alterations. (In addition, research has shown that its measurements are consistently lower than manometric pressure measurements made inside the eye.) So although it’s still the gold standard in the clinic, it may not be the best choice for measuring IOP when a cornea has been altered by pathology or surgery.
If you do use a Goldmann tonometer to measure an eye after corneal surgery, remember that the formula used to adjust readings based on corneal thickness may not apply. As noted earlier, thinner corneas in these eyes may produce falsely low readings, but the reverse isn’t necessarily true if a cornea is thicker as a result of surgery or pathology. For that reason, we don’t recommend using a formula to adjust the measurement based on the corneal thickness.
Pneumatonometer
This instrument (like the Tonopen, discussed below) has a tubular handpiece; the 0.5-mm tip has a fenestrated membrane consisting of a footplate with a microplunger in the center. The IOP equals the pressure required to keep the microplunger flush with the footplate when the tip is in contact with the cornea. (We have a pneumatonometer in our cornea service here at Illinois Eye and Ear Infirmary, which is used to check the IOP after a corneal transplant or in eyes with corneal disease.) This instrument can be used to measure IOP with the patient upright or supine, and it’s not affected by corneal thickness or surface pathology.
One unique thing about this technology is that you can measure the IOP through the sclera. This is an obvious advantage in eyes where you can’t measure the pressure through the cornea, such as eyes with a KPro, a lot of corneal scar tissue or in which the lens and cornea are touching, which can minimize the anterior chamber and make IOP measurements through the cornea inaccurate.
| Corneal changes can affect any tonometry measurement. |
To find out more about this approach, we did a study comparing pneumatonometry readings done through the sclera and in the center of the cornea in normal eyes.4 We found that the scleral and corneal readings were highly positively correlated. However, scleral measurements were consistently higher than corneal readings. We also found that the scleral pressure is less accurate when the IOP is high. At the practical level that means that if you have a patient with a KPro, a low pneumatonometer reading is good for confirming a palpation measurement that suggests IOP is in the normal range.
Tonopen
Some corneal surgeons like the Tonopen because it’s a smaller, handheld device that uses a dis-posable sleeve and only touches a small area of the eye. However, it only has good reproducibility when IOP is in the normal range, from about 10 to 20 mmHg. It underestimates the pressure when it’s higher than that and underestimates it when it’s lower.
If you’re using this device to measure IOP in eyes that aren’t normal, try to stay as close to the center of the cornea as possible. (The cornea is thicker at the periphery, and the Tonopen’s accuracy is affected by corneal thickness—although it’s less affected by corneal edema than some tonometers.) Try to avoid any corneal area that has pathology or a scar. The Tonopen has good reproducibility in the normal range, so a normal reading should be reassuring.
Ocular Response Analyzer
The ORA is a modified air-puff tonometer. It uses an electro-optical sensor to measure the IOP twice, when the cornea is applanated at two different levels, referred to as “bi-directional applanation.” The difference between the two readings is called corneal hysteresis, a measure of viscous damping, which reflects the cornea’s biomechanical properties. (A low hysteresis reading is associated with glaucoma.) The device also measures a “corneal resistance factor” (the cornea’s elastic response), and produces two IOP measures: a Goldmann equivalent and a “cornea-compensated” IOP. Currently, there’s a lot of research going on to expand our understanding of the clinical utility of these measurements.
The advantages of this instrument include that it’s noncontact, and that it measures multiple factors (not all of which we fully understand). Disadvantages include that it’s not available in many practices; it takes two to three seconds to perform a measurement; and multiple measurements are recommended, because they’re affected by the ocular pulse.
Dynamic Contour Tonometer
This instrument’s tip has a concave, 2.5-mm contact surface with a Mylar membrane, a silicone-filled cavity and a piezo-electric transducer. Once the patient’s cornea matches the curve of the tip, the sensor determines the IOP. The device can be connected to a standard slit lamp.
The DCT is thought to produce readings close to the manometric pressure, and in theory, it’s the IOP-measuring device that’s least affected by corneal properties. It also measures ocular pulse amplitude—the difference between systolic and diastolic pressure, which can be useful information—and provides a reliability score for each measurement. Disadvantages include that it takes a little time to get accustomed to using it. Second, the patient has to be very cooperative, because you have to take a number of readings to make sure the measurement is accurate. Third, it’s not readily available in most clinics, so not everyone has access to it.
The Icare Tonometer
This device is a handheld rebound tonometer; its disposable probe accelerates onto the cornea at a fixed speed and a sensor determines how quickly the probe decelerates. (Higher IOP causes a quicker deceleration.) It tends to produce a higher IOP reading than Goldmann tonometry.
Advantages of the Icare include that it’s easy to use; it doesn’t require an anesthetic; it’s well tolerated by children; and it can be used as a home tonometer. It isn’t affected much by corneal edema. Disadvantages include that it may be affected by other corneal biomechanical properties, including corneal thickness, and it tends to be less accurate when the IOP is outside of the normal range.
If using the Icare on a diseased cornea, my recommendation is to avoid areas with pathology but try to stay near the center of the cornea. If band keratopathy is present in the center, it’s better to measure away from the rigid calcified area.
The Big Picture
There are three main things to keep in mind in this situation:
1. Corneal changes can affect any tonometry measurement, although the amount of impact is different from one device to the next. Goldmann tonometry is most affected by corneal changes; the Dynamic Contour Tonometer is least affected. Also, ac-curacy is often highest when the IOP is in the normal range.
2. If you know the cornea you’re measuring isn’t normal, you might
want to use an alternative to Goldmann, such as the Pneumatonometer, which can measure through the sclera, or the ICare or Tonopen. (Both of these produce more accurate readings near the center of the cornea and away from pathology.)
3. Don’t switch between instruments when monitoring a given patient over time. Measurements from one device to the next don’t usually match. REVIEW
Dr. Vajaranant is an associate professor of ophthalmology at the University of Illinois College of Medi-cine at Chicago and director of the Glaucoma Service at the Illinois Eye and Ear Infirmary. She has no financial interest in any of the pro-ducts mentioned.
For more information, check out: Desai M. Intraocular Pressure Mea-surement. In: Edward DP, Vajaranant, TS, eds. Glaucoma. Oxford American Ophthalmology Library. Oxford University Press 2013:1-14.
1. Vajaranant TS, Price MO, Price FW, Wilensky JT, Edward DP. Intraocular pressure measurements following Descemet stripping endothelial keratoplasty. Am J Ophthalmol 2008;145:5:780-6.
2. Baum J, Chaturvedi N, Netland PA, Dreyer EB. Assessment of intraocular pressure by palpation. Am J Ophthalmol 1995;119:650-51.
3. Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology 1998;105:751-57.
4. Kapamajian MA, de la Cruz J, Hallak JA, Vajaranant TS. Correlation between corneal and scleral pneumatonometry: an alternative method for intraocular pressure measurement. Am J Ophthalmol 2013;156:5:902-906.e1



