The retina and optic nerve receive the lion’s share of attention when it comes to glaucoma, but an increasing amount of evidence suggests that brain function may have some role to play as well. One potential vision therapy, known as repetitive transorbital alternating current stimulation or rtACS, delivers electrical impulses to the brain, aiming to modulate damaged brain networks and improve low vision. Early studies show promising results. Here, experts explain how this technology works, what it means for glaucoma treatment, and how reducing stress may lead to better outcomes.
Residual Vision
 |
|
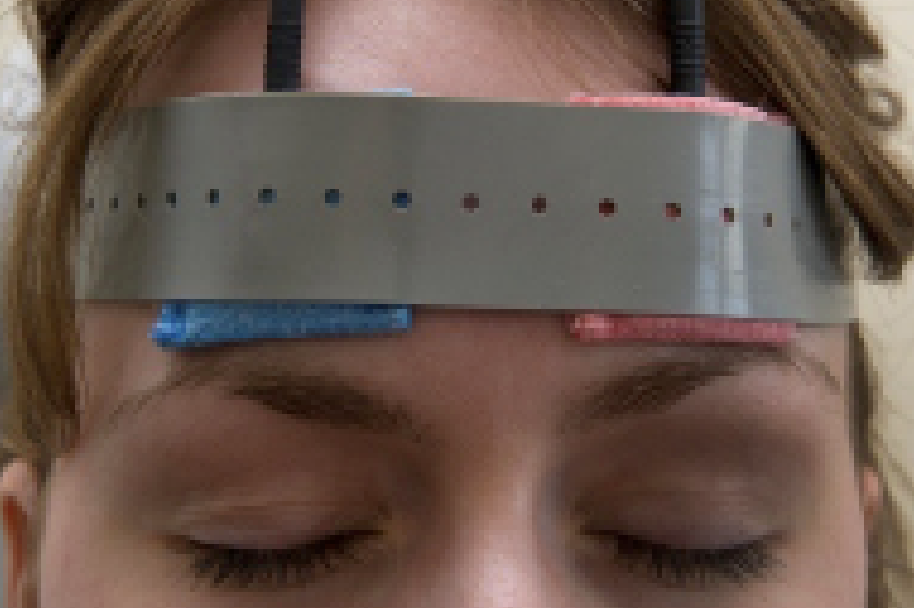
The repetitive transorbital alternating current stimulation device on a glaucoma patient. Therapy is administered for 10 days. All images: Bernhard Sabel, PhD. |
“The concept behind rtACS is that there are nerves—ganglion cells—that are dead in glaucoma, but then there’s a penumbra of surrounding ganglion cells that may be injured but not dead,” says Joel Schuman, MD, of Wills Eye Hospital in Philadelphia. “Neurostimulation enhances the function of those injured retinal ganglion cells.”
“We refer to these as ‘silent neurons,’ ” says Bernhard Sabel, PhD, of Otto-von-Guericke University of Magdeburg in Germany, who first proposed the idea of residual vision activation in 20111 and back in 1998 demonstrated that computer-based visual training could improve visual field defects resulting from brain injury.2 “Neurons can fall into an inactive state due to damage from long-term or sudden vasoconstriction. Reactivating these silent neurons to improve vision can be achieved by electrical stimulation, which mimics the brain’s electrical impulses, helping the brain’s functional networks to reorganize themselves in a more normal structure.”
The human brain is remarkably adaptable. “The brain has billions of nerve cells and they’re all connected to each other functionally,” explains Dr. Sabel. “Neural signals can travel from anywhere in the brain to any other place in the brain by just four or five synaptic connections. A hundred billion nerve cells with 10,000 synapses make for a lot of options when forming functional connections. Of course, these only travel along the anatomical connections in the brain.
“A functional connection is the path that one nerve signal travels to reach its target,” he continues. “It could take a direct route—a one-to-one anatomical line from point A to point B—but it typically doesn’t. Usually, neural signals jump across several synapses to reach their target. When you have damage—dead or injured neurons—it creates a hole in the network and forces the information to travel on a different route. The damage produces desynchronization, or a disturbance of the brain network.”
In studying functional brain connectivity features in partially blind patients with peripheral optic nerve lesions treated using rtACS,3 Dr. Sabel and his colleagues found that visual field improvements were associated with resynchronization of alpha band coherence, an electroencephalogram rhythm that regulates the functional connectivity between brain regions.
This reorganization of brain networks, also called neuromodulation, was also noted in optic neuropathy patients who underwent rtACS therapy.4 The multicenter, prospective, randomized, double-blind study included 45 rtACS-treated patients and 37 sham-stimulation patients. Each underwent daily treatment or sham treatment for 50 minutes over a 10-day period. At two months, the rtACS-treated group showed a mean visual field improvement of 24 percent, compared with a 2.5-percent improvement in the sham-stimulation patients. Near-threshold visual fields in the central five degrees also improved, along with thresholds in static perimetry and reaction times after treatment. No visual acuity changes were noted in the treatment vs. sham group. The researchers noted that visual field improvements from rtACS were associated with electroencephalogram power-spectra and coherence alterations in visual cortical networks, which are considered signs of neuromodulation. They concluded that the treatment was safe and effective for partial vision restoration after optic nerve damage and worked presumably by modulating brain plasticity.
The rtACS therapy was also reported to improve visual and cognitive deficits in two patients suffering from long-COVID.5 An analysis of retinal vessels showed reduced vascular dysregulation after treatment, in addition to partial reversal of visual field loss and improved cognitive subfunctions. Since SARS-CoV-2 infection has been shown to cause reduced capacity for blood flow,6 the researchers propose that recovery was linked to restoration of vascular autoregulation and subsequent reoxygenation of neurons, though further research is needed.
Dr. Sabel says that improving vascular regulation is an important component of rtACS therapy. He points out that “restoration of the injured neurons requires the return of sufficient blood supply, which is achieved in part by muscle relaxation and subsequent vasodilation from electrical stimulation and by stress reduction.”
 |
|
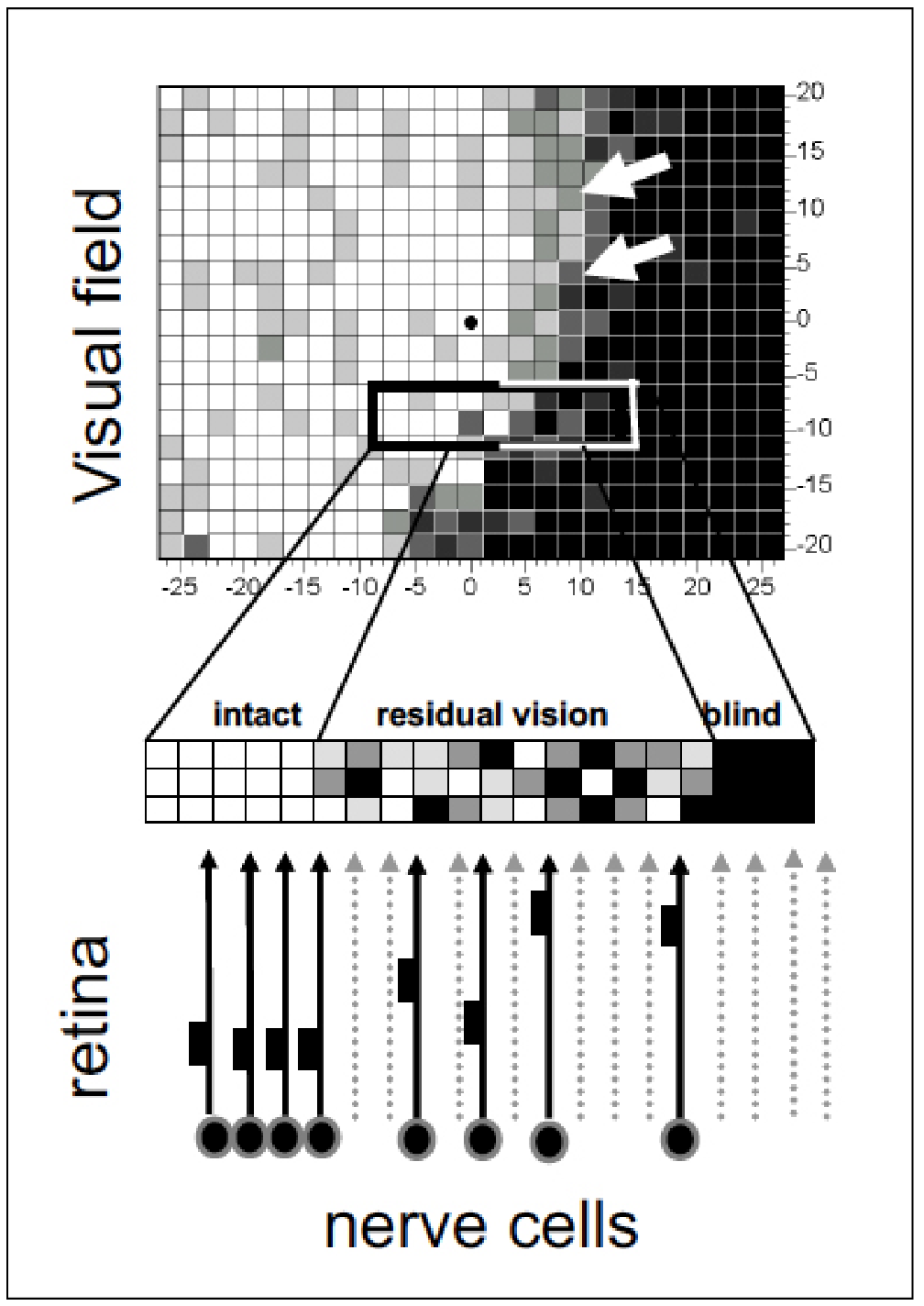
Neurostimulation research has shown that it's not necessarily an all-or-nothing situation with neurons. Some may be damaged rather than dead. These "silent" neurons (areas of residual vision) are the target of rtACS treatment. |
Repetitive Transorbital Alternating Current Stimulation (rtACS)
The rtACS device produces a mild electrical current that’s transmitted to the body through the orbit. “There are little pads that are in contact with the skin above the orbit,” Dr. Schuman explains. “That electrical stimulation is believed to stimulate the retina, optic nerve and anterior brain to improve their function. The device was developed by Bernhard Sabel, PhD, in Germany and we’re collaborating with him on our studies. We had two different studies at NYU. One was a randomized controlled clinical trial with 15 subjects.”7
The subjects were randomized to either a treatment group or a sham group whose subjects thought they were receiving treatment but were not. (This was achieved by tuning the device as if subjects were going to receive treatment, to a level that yielded phosphenes, and then turning the device down to a level at which subjects wouldn’t feel anything, Dr. Schuman explains.)
The treatment consisted of 10 approximately 45-minute sessions over a two-week period, where subjects would come into the office every weekday to receive treatment. “What we found was a trend, but not a statistically significant finding, for improvement in visual field,” Dr. Schuman says. “There was a statistically significant finding for improvement in quality of life in the treatment group vs. sham. I’d say this is encouraging. We measured several different quality-of-life parameters, using a comprehensive combination of those parameters.”
Dr. Schuman’s group also ran an expanded access or compassionate use study, where all subjects knew they were receiving the treatment. “These were patients who didn’t qualify for the pilot study,” Dr. Schuman explains. “We treated around 70 people. What we found was that both in the pilot study and in the expanded access study, there were certain people who responded with an improvement in visual fields.
“This improvement had a characteristic pattern, where there would be a steep improvement in mean deviation over several tests and then a slowing of that improvement and stabilization,” he continues. “After a certain amount of time—which could be months or years—most of these patients who saw improvement experienced a decline of that improvement back to baseline. If they were retreated at that point, these patients sustained a similar improvement to that of the initial treatment.”
He says it’s unclear why these patients in particular improved. “We haven’t done that analysis yet, but it would certainly be good to know so that we could predict who would have a significant benefit from the treatment,” he says.
Of the patients who noticed an effect of the treatment, Dr. Schuman says they would describe an improvement in the area of “glaucoma fog,” that the fog had cleared in a certain area. “Now, these were patients who had knowledge that they were actually receiving treatment,” he notes. “I have to wonder if there’s bias because of that expectation of improvement. The randomized controlled pilot study is much more convincing in terms of the potential benefit of this treatment. But the expanded access study allowed us to look at more people, and there were spontaneous expressions of what the subject was experiencing. I thought that was an interesting description of their subjective experience following treatment.”
He adds that some subjects noticed an improvement in their perception of colors and said things looked brighter. “I would say those were fewer—it was mostly the glaucoma fog,” he says. “If subjects noticed an improvement, it was usually described in that way.”
What does neurostimulation research add to our understanding of glaucoma pathogenesis? “I think it helps us understand that not all neurons that aren’t functioning properly are dead,” Dr. Schuman says. “There may be some small fraction of the damaged neurons that we’re able to recover.” He emphasizes that this form of neuro-enhancement shouldn’t be confused with neuro-regeneration. “I think that’s an important distinction. We aren’t actually bringing any neurons back to life.”
Dr. Schuman says it’s important to point out that there’s currently no well-established treatment that improves vision in people with glaucoma. “While we only saw a trend in our small pilot study toward visual field improvement, we did have individuals in whom we noticed this type of improvement that I described. And nothing does that. I think that’s very encouraging, and it’s grounds for further larger studies of this technology. There’s a home version of this technology that’s currently being tested. NYU set up a study to look into it with Stanford, and Wills will soon join that collaborative study.
“I think that if it turns out that our preliminary findings and the findings that Dr. Sabel reports are reproduced, or they’re expanded, this could easily find its way toward clinical treatment,” he says. “It’s basically a risk-free treatment, and it may enhance glaucoma patients’ vision. It seems to statistically significantly improve their quality of life, so I would be optimistic about it becoming a clinical tool.”
 |
|
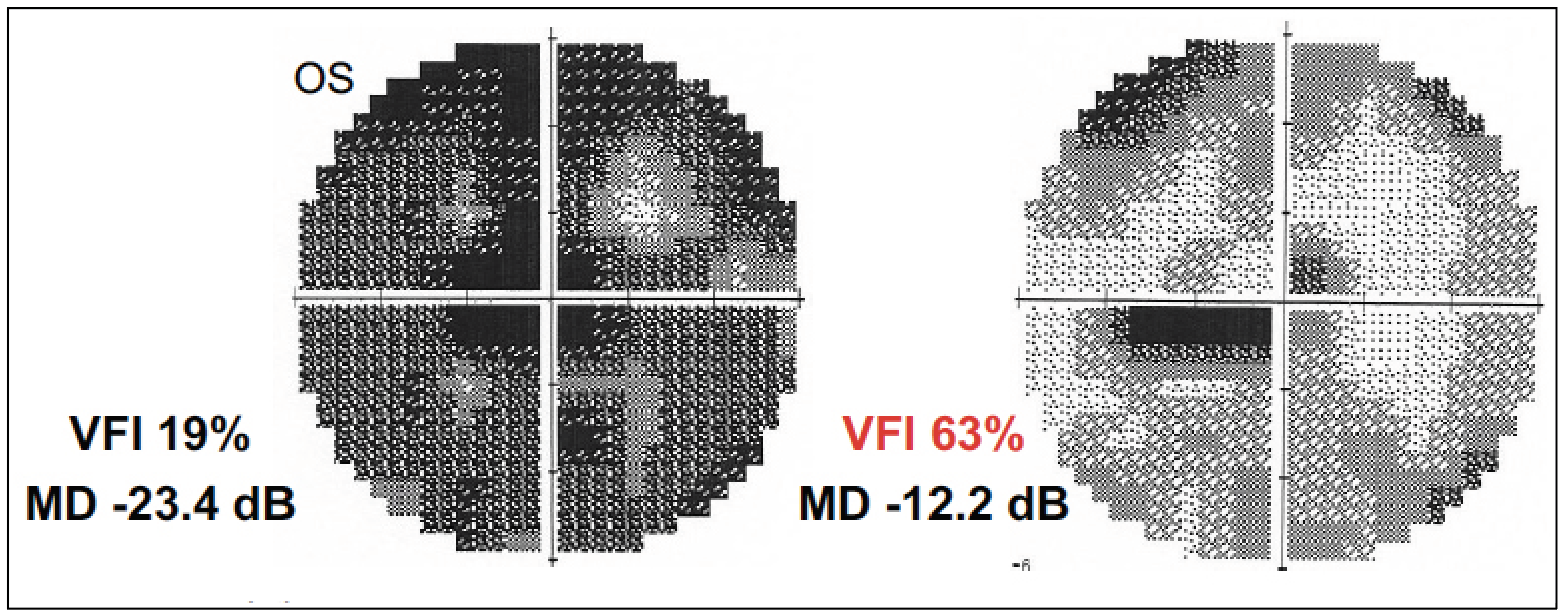
Visual field improvement in a 52-year-old female patient with primary open-angle glaucoma after a 10-day course of daily 20- to 40-minute rtACS sessions at <2 mAmp, 5-25 Hz. |
In Early Clinical Trials
An at-home rtACS device is currently in clinical trials. Study investigator Jeffrey L. Goldberg, MD, of Stanford University, writes in an emailed comment, “Dr. Sabel has shown efficacy over the years using a two-week (10-day) course of electrical stimulation to improve patients’ vision or visual fields. This multicenter trial is designed to test whether a two-month course of self-treatment at home would work similarly, or even work better than these prior approaches.”
The clinical trial at Stanford and NYU is currently recruiting study participants to test the safety and efficacy of long-term rtACS therapy with an at-home device in patients with open-angle glaucoma (NCT05626491). A study for optic neuropathies (NCT05626426) is also recruiting at Stanford. The randomized, double-masked glaucoma study involves active or sham treatment with the device every other day over eight weeks. Estimated enrollment is 45 patients. Primary outcome measures include six-month change from baseline in visual field assessed by Humphrey Visual Field Index. Secondary outcome measures include six-month changes from baseline in visual field assessed by Humphrey mean deviation, by Pelli-Robson contrast sensitivity and by Snellen visual acuity.
The Stress Factor
Dr. Sabel has treated more than 2,000 patients over the last decade with this neurostimulation therapy at the Savir-Center in Magdeburg, Germany. He says that improvement asymptotes and reaches a ceiling, so in general patients with moderate or severe disease will gain more benefit than a patient with minimal vision loss. However, response to treatment varies considerably.
“A regular question I get from patients is ‘how much will it improve my vision?’ We don’t make those predictions,” he says. “Even a very small change could be subjectively meaningful to the patient. We’ve found that the patient’s response and how much they improve depends not only on the electrical stimulation they receive but also on mental attitude.”
According to Dr. Sabel, of the patients treated for a 10-day course at the Savir-Center, about 85 percent show visual function improvement and about 15 percent benefit very little or not at all. He attributes these outcomes to variations in mental stress.
How does stress influence the amount of residual vision a patient has or gains back? “Most patients always have some residual vision, but stress can reduce the amount,” claims Dr. Sabel. “The influence of stress is two-fold. The sympathetic nervous system activates the body’s fight-or-flight response, and the body undergoes a typical stress response: muscle tension; increased attention; increased blood pressure; and adrenaline release. The parasympathetic nervous system reduces activity. When this system dominates, the body is calmer with less muscle tension, normalized vessel constriction and activated gland activity. Meditation and breathing exercises can bring the two systems back into balance. Remaining in an overstressed state is unhealthy, and I believe it contributes to glaucoma. Vascular dysregulation is a key feature of glaucoma in addition to neuronal health. Vasoconstriction results in decreased blood flow and oxygen availability to nerve cells, often causing the nerve cells to go into a quiet, inactive state.”
In a prospective trial of 90 POAG patients (180 eyes) randomized to a daily meditation group for 21 days or a waitlist group, mindfulness-based stress reduction was found to significantly lower IOP, improve quality of life (WHO QOL questionnaire) and normalize typical stress biomarkers, including cortisol, IL-6, TNF-alpha, reactive oxygen species, beta-endorphins, brain-derived neurotrophic factor and total antioxidant capacity (all p<0.001). Additionally, positive parallel changes in gene expression were also observed, leading the researchers to conclude that mindfulness meditation is a useful adjunctive therapy in glaucoma.8
Dr. Schuman notes that further study is needed to determine the role stress plays on glaucoma. “We don’t know much about the effect emotional stress has on glaucoma or the perception of people who have glaucoma,” Dr. Schuman says. “I don’t negate it, but we need more information.”
Relaxation Techniques
Dr. Sabel is currently involved in a controlled trial (NCT04037384) to compare what he calls eye yoga, which includes eye movement exercises and meditation, for four weeks vs. active control (passive reading). Estimated enrollment is 40 participants, randomized to treatment or control arms. Primary outcome measures include visual field change, evaluated by static perimetry and high-resolution perimetry and changes in deviation of eye movements when visual targets are followed, using a computerized test. Secondary outcome measures include changes in the diameter of ocular blood vessels measured using vessel analysis and changes in alpha power using EEG recording (128 channels).
“We adopted meditation techniques and offer them to our patients now in conjunction with electrical stimulation therapy, which is the double-punch to increase blood vessel diameter and get the neurons firing again,” he continues. “Yoga is a way of life, not just a series of asanas or poses, and I call this eye yoga—the combination of eye exercises plus meditation. Along with psychological consulting aimed at improving stress resilience, we support patients to live a more relaxed life overall.”
He explains that the eye exercises involve moving the eye right, left, up, down and in circles. He also recommends palming (covering both eyes and then removing the palm to induce pupillary contraction), gentle massage around the eye, and massaging the neck. “The neck’s very important,” he says. “Tension in the neck and head region can lead to headaches and can be bad for vision.
“As you might imagine, these relaxation techniques only work if the patient isn’t afraid,” Dr. Sabel points out. “Patients who constantly dwell on the idea of going blind, losing vision if they don’t do x, y and z, or just on the fact that they have a progressive sight-threatening disease, will find it more difficult to relax and release stress.”
For this reason, he says it’s important that clinicians focus on positivity during patient interactions. “Glaucoma is well-known as a sight-threatening condition, and treatment is aimed at slowing its progression,” he says. “Patients take their drops in order to avoid losing more vision, but to patients, this often translates as ‘to avoid going blind.’ Therefore, communicating with the patient in a manner that emphasizes the positive is important to alleviate fear and reduce patient stress.”
While it’s very rare for individuals to have zero light perception, this is often what patients associate with blindness. “Usually, ‘blind’ for a patient means black-blind in both eyes and no vision whatsoever,” Dr. Sabel says. “Tell patients, ‘You will not go blind.’ Even if a doctor doesn’t explicitly tell a patient they could or will go blind, patients often have this intense fear. What you say isn’t always what the patient hears, and what the patient hears, they don’t necessarily understand or even agree with. When patients are afraid and have a negative outlook, they’re more likely to have anxiety about their condition. They may stay home more often and become socially isolated. All of this creates stress. It’s a massive mental relief to patients when you reassure them that they won’t go blind. Barring very severe cases, delivering this optimistic message will do more good than harm.
“Ophthalmologists don’t have a lot of time to talk with their patients,” he acknowledges. “They may see 100 patients in a day, and don’t have psychologists in their office who can talk about vision loss, stress or quality of life issues with patients. This, we may not be able to change. But ophthalmologists can try to allay fears by approaching patients with confident, positive attitudes. In a nutshell, my recommendation would be: Tell patients that the future outlook is optimistic. Say, ‘Here’s the treatment plan to preserve the vision you have. Take your drops, drink enough water, try to relax and not get yourself stressed out.’"
Dr. Sabel is the creator of the rtACS device in clinical trials and the founder of the Savir-Center in Magdeburg, Germany. Dr. Goldberg and Dr. Schuman have no related financial disclosures.
1. Sabel BA, Henrich-Noack P, Fedorov A, Gall C. Vision restoration after brain and retina damage: The “residual vision activation theory”. In: Andrea M. Green, C. Elaine Chapman, John F. Kalaska, and Franco Lepore, eds. Progress in Brain Research vol. 192—Enhancing performance for action and perception: Multisensory integration, neuroplasticity and neuroprosthetics, Part II. New York: Elsevier, 2011:199-262.
2. Kasten E, Wüst S, Behrens-Baumann W, Sabel BA. Computer-based training for the treatment of partial blindness. Nature Medicine 1998;4:1083-1087.
3. Bola M, Gall C, Moewes C, et al. Brain functional connectivity network breakdown and restoration in blindness. Neurology 2014;83:542-551.
4. Gall C, Schmidt S, Schittkowski MP, et al. Alternating current stimulation for vision restoration after optic nerve damage: A randomized clinical trial. PLoS One 2016;11:6:e0156134.
5. Sabel BA, Zhou W, Huber F, et al. Non-invasive brain microcurrent stimulation therapy of long-COVID-19 reduces vascular dysregulation and improves visual and cognitive impairment. Restorative Neurology and Neurscience 2021;39;6;393-408.
6. Ahmed AI, Saad JM, Han Y, et al. Coronary microvascular health in patients with prior COVID-19 infection. JACCCardiovasc Imaging 2022;5:12:2153-2155.
7. Sohn A, Cadena M, Lee T, et al. Effect of repetitive transorbital alternating current stimulation (rtACS) on vision-related questliy of life (QoL) in subjects with glaucoma. Invest Ophthalmol Vis Sci 2023;64:4351.
8. Dada T, Mittal D, Mohanty K, et al. Mindfulness meditation reduces intraocular pressure, lowers stress biomarkers and modulates gene expression in glaucoma: A randomized controlled trial. J Glaucoma 2018;27:1061-1067.