In the coming year, you’ll be able to prescribe a new daily disposable contact lens, Precision1, that its manufacturer, Alcon, says is ideally suited to the first-time contact lens wearer. Research cited by the company shows that 20 percent of new wearers drop out within the first year, and 57 percent don’t inform their eye doctor when they do.
To help, Alcon developed Precision1 to negate what it identifies as the top three motivators of drop out: poor vision; poor comfort; and even the frustrations that arise from poor lens handling.
 |
Precision1 uses a new silicone hydrogel material, verofilcon A, and includes a permanently adhered ‘microthin’ (2 to 3 µm) layer of moisture. Alcon says this feature, which it calls SmartSurface, improves comfort and supports a stable tear film. The lens has a water content of 51 percent at the core and greater that 80 percent at the anterior surface.
The company says the lens will be designed to be a mid-tier option between its Dailies Aqua Comfort Plus and Dailies Total1 lines of contact lenses. The lens will be available in a power range of -12 D to +8 D, with a 14.2-mm diameter and an 8.3 base curve. Precision1 will begin rolling out to select doctors in the United States in September, with widespread access anticipated for early 2020. For information, visit alcon.com.
New Prefilled Syringe for Aflibercept Approved by FDA
Regeneron Pharmaceuticals recnetly announced the U.S. Food and Drug Administration approved the Chemistry, Manufacturing and Controls Prior-Approval Supplement for the Eylea (aflibercept) Injection prefilled syringe.
The 2 mg, single-dose, sterilized prefilled syringe provides physicians with a new way to administer Eylea that requires fewer preparation steps compared with vials. The company says that it expects the prefilled syringe to be available to physicians this year.
For information on the prefilled syringe for aflibercept, visit https://investor.regeneron.com/news-releases/news-release-details/fda-approves-eylear-aflibercept-injection-prefilled-syringe.
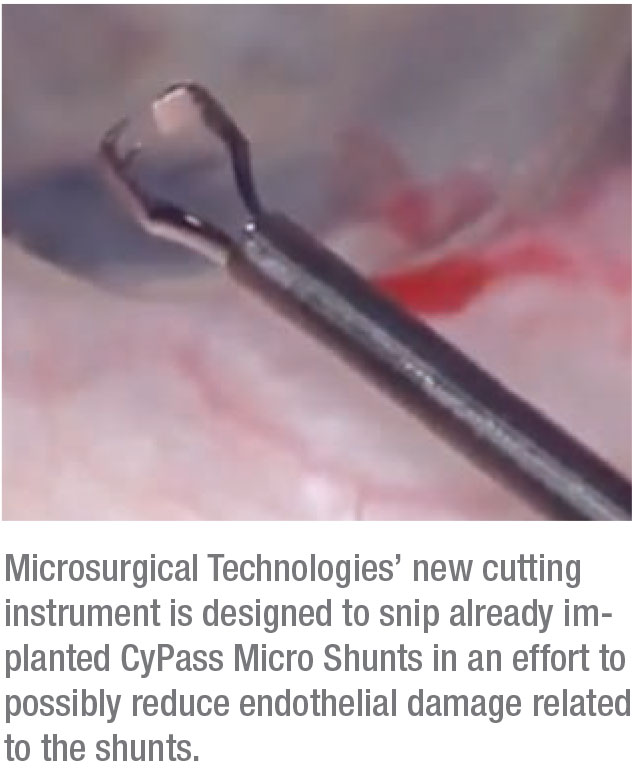 |
Help for CyPass Patients
If you’ve got patients with Alcon CyPass glaucoma stents in their eyes that need revision, Microsurgical Technologies says its new instrument, the 19g Ahmed Micro Stent Cutter, might be of use.
MST says that it developed the cutter in collaboration with Toronto surgeon Ike Ahmed, MD, and says the unique design of the instrument enables surgeons to approach a micr-stent coaxially, grasp and trim a stent in a single step using only one hand and make a clean cut of the stent’s proximal end.
For information on the new cutting instrument, visit https://microsurgical.com/.
PanOptix Trifocal IOLs Now Available for U.S. Patients
Alcon’s AcrySof IQ PanOptix trifocal IOL has been granted FDA approval. It’s the first and only FDA-approved trifocal lens for U.S. patients, says the company. The PanOptix is a one-piece aspheric hydrophobic presbyopia-correcting IOL1 built on the AcrySof IQ platform that uses Enlighten optical technology, Alcon’s proprietary design for optimizing intermediate vision. Its intermediate focal point is slightly closer than traditional trifocal IOLs at 60 cm,1 which is considered a more comfortable and natural working distance for performing technology-related tasks.1
Achieving good intermediate visual acuity is especially important in today’s technology-driven world, where using screens is part of daily life. “Until now, my patients were forced to choose between near and intermediate vision,” says Dagny Zhu, MD, in practice in Rowland Heights, CA. “But both are crucial for patients who lead active lives. I can offer them so much more now.”
According to a 2017 meta-analysis literature study comparing the clinical performance of multifocal and trifocal IOLs, trifocals outperformed multifocals in intermediate visual acuity.2 A 2018 study of the same kind conducted in Seoul also found that trifocals outperformed multifocals in intermediate visual acuity and provided similar or better distance and near visual acuity without compromising visual quality.3
Dr. Zhu is excited about the reduced glare and improvement in contrast vision that comes with trifocals. “I think surgeons who have been hesitant to offer multifocal and extended depth of field lenses in the past will be more willing to try the trifocal lenses,” she says.
Alcon says the PanOptix trifocal lens significantly reduces the need for glasses after surgery. PanOptix is available in both spherical and toric designs. The company plans to host special launch activities at the 2019 AAO meeting in October.
For information, visit alcon.com. REVIEW
1. Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. AcrySofIQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: A clinical overview. Asia Pac J Ophthalmol (Phila) 2019 Jul-Aug;8:4:335-349. PMID 31403494
2. Xu Z, Cao D, Chen X, et al. Comparison of clinical performance between trifocal and bifocal intraocular lenses: A meta-analysis. PLoS One 2017 Oct 26;12:10:e0186522. PMID 29073156
3. Yoon CH, Shin IS, Kim MK. Trifocal versus bifocal diffractive intraocular lens implantation after cataract surgery or refractive lens exchange: A meta-analysis. J Korean Med Sci 2018 Sept 27;33:44:e275. PMID 30369857




