The number of minimally invasive glaucoma surgeries performed is increasing every year. Combined with cataract surgery, MIGS offer additional pressure lowering, can reduce patients’ medication burden and also decrease the risk for postop pressure spikes. While complications can occur with the addition of a second procedure to cataract surgery, in the hands of an experienced surgeon, this risk is low.
Here, I’ll share some pearls to set surgeons up for success with angle-based surgery regardless of technology.
Gonioscopy
Visualizing surgical landmarks is key. Before incorporating MIGS, practice careful gonioscopy in the clinic to get to know the angle anatomy. Once you’re comfortable with that, practice gonioscopy in some of your routine OR cases to learn how to find the best view and get used to handling the gonio lens with your non-dominant hand.
Avoid pressing too hard on the eye with the gonio lens as this can create striae. Match the scope tilt with how much you’ve turned the patient. The scope tilt should offer a direct, perpendicular view of the angle.
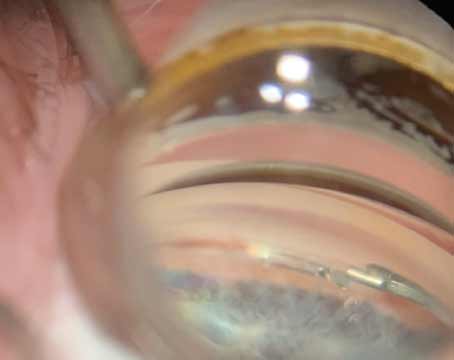
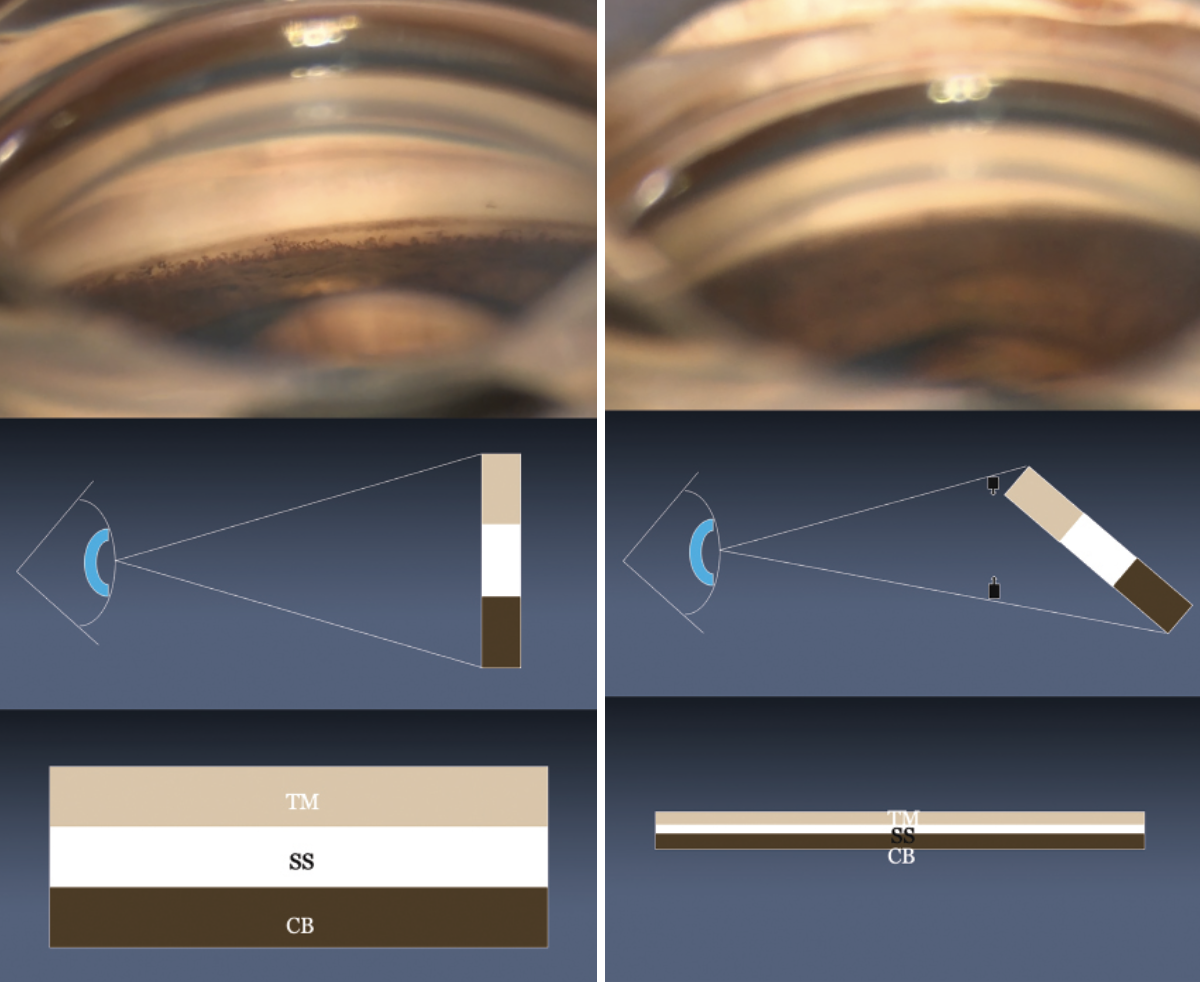 |
| Figure 1. (A) An en face view of the angle with visibly distinct structures. (B) Under-rotation is a common reason for a reduced surgical view. Here, the perceived height of the angle is decreased. |
Parsing the En-face View
The en face, or forward-facing, view is relative to the observer. In the view shown in Figure 1A, the angle is en face relative to the surgeon, and the structures of the angle—trabecular meshwork, scleral spur and ciliary body band—are clearly differentiated.
As the patient’s head rotates toward the surgeon, the perceived height of the angle shortens and the perceived relative distance between the trabecular meshwork and ciliary body band is reduced (Figure 1B). Under-rotation is the most common reason why our surgical view is reduced during angle surgery and it’s the major mechanism by which clefts occur during goniotomy.
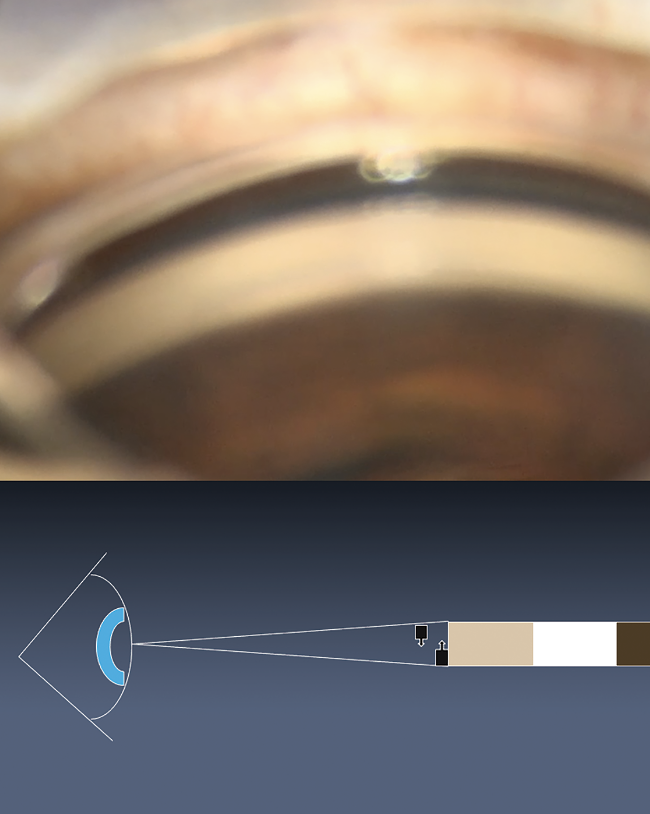 |
| Figure 2. In an extreme example of the eye turned completely toward the surgeon, no distinct angle structures are visible. |
With this view, the surgeon can’t determine what layers they’re seeing. The ciliary body band looks relatively larger. When we look to treat the band of pigment, we might be in the ciliary body band instead. The trabecular meshwork sometimes looks fused with the ciliary body band, especially when the layers are less distinct with pigment confluent throughout, as we see in some angles.
To further illustrate with an extreme of this example, if the eye is turned completely toward the surgeon, the trabecular meshwork appears directly in line with the ciliary body band (Figure 2). In this view, there are no distinct structures. The view depicted in Figure 1A is what we expect to see on gonioscopy.
Use Trypan Blue
One of the reasons for improper device placement is looking for pigment and not looking for the slight translucency of the canal. Document trabecular meshwork pigmentation during your preoperative evaluation. If the pigmentation is very light, be sure to make a note in the chart to use trypan blue (Figure 3). Inject the dye at the beginning of the case, ensuring that it gets into the peripheral angle. Adding intracameral lidocaine can help get the dye into the peripheral trabecular meshwork.
 |
| Figure 3. Injecting trypan blue at the beginning of the case will stain the trabecular meshwork, making it easier to visualize the angle structures. |
Controlling Heme
If you encounter bleeding while creating your incision, use your second hand or have an assistant wick it away with a Weck-Cel sponge (Figure 4). Maintain control of bleeding during and especially post-procedure (more on that below). Don’t pull the gonio prism on and off. This can create a suction effect that draws more blood into it. Making the incision slightly more anterior can avoid additional bleeding.
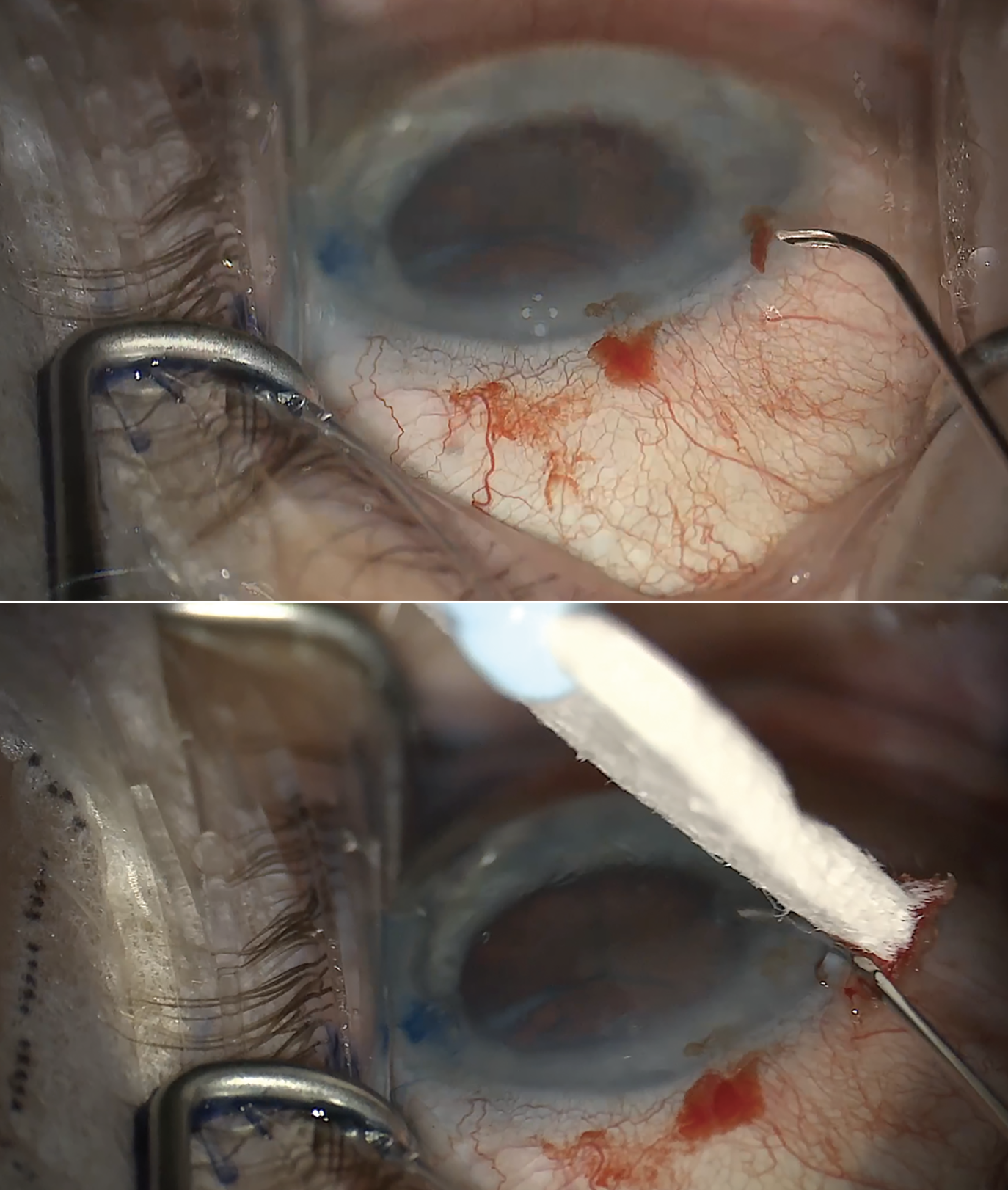 |
| Figure 4. If bleeding occurs during incision creation (A), use a Weck cell sponge to clean up the area (B). At the end of the case, raise the pressure to approximately 25 mmHg and irrigate the blood before letting the patient go. Postoperatively, patients with a significant amount of reflux should stop their glaucoma drops day-of. However, continuing drops is recommended for a patient with severe disease and little reflux. |
Placing Devices
Avoid overfilling the eye with OVD, as this can compress Schlemm’s canal and make it more difficult to place devices. An exception would be if you’re performing a pressurized viscodilating procedure; higher pressures can limit Descemet’s detachments. Underfilling the eye may cause the iris to bow forward and obscure your view of the angle.
As you’re placing devices, don’t push too hard. With stents in particular, the initial portion of device placement involves pushing forward to incise into the trabecular meshwork and holding the tip of the cannula against the back wall of Schlemm’s canal. When the first window of the stent is in the canal, particularly with Hydrus or iTrack, you’ll want to back the pressure off in order to avoid driving the device more posteriorly.
If the device meets resistance, make space by creating a micro goniotomy. Be sure to place the device about a millimeter just before the goniotomy entry site instead of right at the entry site. Dock the device against the back wall. This allows you to float the device into the angle. Here are a few ways to do this:
1. Use the injector to create a goniotomy by scratching off the trabecular meshwork (Figure 5).
2. Use a bent 25- or 27-gauge 5/8ths-inch needle to make a scratch incision (Figure 6).
3. Use OVD to open up the channel. Create a small goniotomy and dock a viscoelastic cannula up against it. Push against the back wall and inject a small amount of OVD just to inflate the tissue plane in front of where you want to go.
Layers of the Trabecular Meshwork There are multiple tissue planes that devices can be laid into. These include: |
Postoperative Care
Be sure to follow the postoperative course to determine whether or not the pressure is responding. Look carefully at device placement. If a stent isn’t in the right spot, it’s not going to work. Especially in some tighter angles, patients could end up with PAS. Larger devices can sometimes cause a little bit of inflammation if they’re chronically rubbing against the posterior iris.
There are some angles that you won’t be able to get devices in. If you’re not satisfied with the device placement, remove the device. Don’t leave the device in the wrong tissue plane just to have it in the eye. Setting expectations about this potential outcome before surgery can ease patient concerns.
In the postoperative period, it’s also important to manage bleeding. If there was a lot of bleeding during the surgery, then you can consider leaving a little bit of OVD, but we’ve gone away from using viscoelastic in the eye and moved toward raising the pressure at the end of the case to about 25 mmHg. Be sure to irrigate the heme before letting the patient go.
Most patients should stop their glaucoma drops on the day of the surgery, especially if they get a significant amount of reflux. If you don’t see much reflux in a patient with severe disease, I’d recommend continuing with the drops.
 |
| Figure 5. If you encounter resistance upon device insertion, one option is to create a micro goniotomy using the injector tip. |
 |
| Figure 6. A bent 25- or 27-ga. needle creating a scratch incision to make space for device insertion. |
Tackle the TM First
While surgeons will have their own preferences and comfort levels when it comes to combined surgery, there are a number of advantages to performing a trabecular meshwork-based surgery before phacoemulsification. Here are a few reasons to consider:
• If you’re confident in your cataract surgery and less used to the angle procedure, start with the angle procedure to get it out of the way.
• Patients undergoing combined surgery are at high risk for pressure spikes after cataract surgery. In the event that you break the capsule while performing cataract surgery first, you probably won’t continue with the angle procedure. Performing the angle procedure first gives you at least some backstop of addressing pressure should a cataract surgery complication occur.
• Visualization may be improved during phaco since OVD can deepen the angle. Lens removal isn’t necessary to see the angle—if you’re in that position, with a tight plateau-like configuration, then you probably shouldn’t be placing a device in that patient anyway.
• The I/A during the angle procedure helps to pressurize the system, removes blood reflux as you phaco and allows for less heme at the end of the case.
When performing angle surgery first, be sure to manage the heme. Keep the chamber pressurized to avoid reflux. Blood can sometimes get into the capsular bag, depending on the zonular integrity, and even the posterior segment.
In summary, the best way to practice angle surgery is to do it. Familiarize yourself with angle anatomy. Document the trabecular meshwork pigmentation, using trypan blue to improve visualization if needed. Maintain careful control of heme perioperatively and consider performing the angle-based procedure before phaco. Avoid overfilling the eye with OVD, except in cases of pressurized viscodilation. If the device meets resistance during insertion, make space with a micro goniotomy. Finally, don’t hesitate to remove the device if it’s in the wrong tissue plane.
Dr. Sheybani is an associate professor of ophthalmology and visual science at the Washington University in St. Louis School of Medicine. He’s a consultant for AbbVie, Alcon, Nova Eye and Glaukos, and an ad hoc consultant for Santen and New World Medical.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.