The right ciliary body ablative procedure is a valuable tool for managing pressures in glaucoma. Endoscopic cyclophotocoagulation and micropulse cyclophotocoagulation are gentler approaches that may be used in mild to moderate glaucoma. Traditional transscleral cyclophotocoagulation is generally considered more effective at pressure lowering, depending on the settings used, but is most commonly reserved for patients with poor vision (e.g., count fingers at one inch) and later-stage disease.
There are numerous indications for ciliary body ablation. Here, I’ll review scenarios where ciliary ablation may be beneficial, discuss the best laser settings to use and share tips for success.
Additional Subconjunctival Surgery or Cycloablation?
In certain situations, performing another filtering procedure may be unpalatable to the patient or may put them at additional risk for complications. For patients in their 90s, for example, my interest in doing an incisional procedure is lower because they have a higher risk of suprachoroidal hemorrhage. Ciliary ablation may be a good option for such patients.
Here are some more examples of patient scenarios suited to a ciliary body ablative procedure:
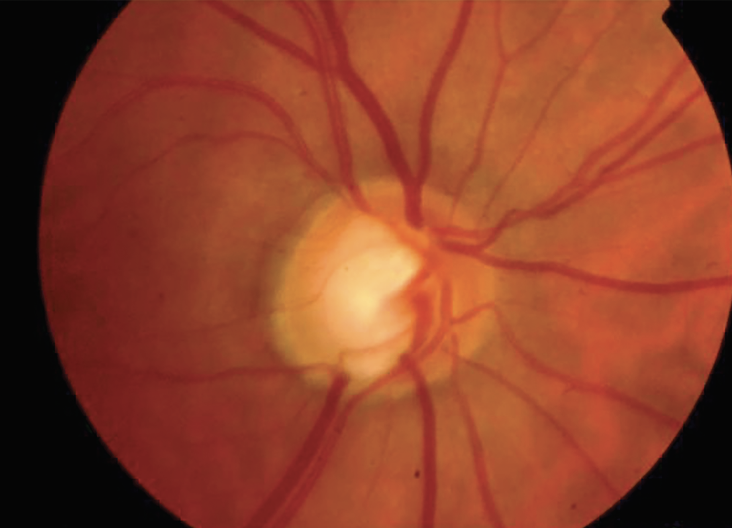 |
| Figure 1. A 60-year-old African American male patient with open-angle glaucoma in the right eye is allergic to multiple drops. His surgical history includes trabeculectomy, bleb revision x2 and Ahmed Clearpath 350 implantation. Best-corrected visual acuity is 20/25. His T-max is 23 mmHg and his pressures are persistently in the high teens on a preservative-free PGA. Target IOP is 14 mmHg. A 360-degree ECP was completed with two incisions. At postop month four, his IOP was at the target of 14 mmHg on a preservative-free prostaglandin analog. Photo: Osamah Saeedi, MD, MS. |
• Failed tube. After a failed tube shunt surgery, patients may not wish to undergo an additional tube shunt surgery or deal with potential tube-related complications such as choroidal hemorrhage (Figure 1). While ciliary body ablation has associated risks of its own (discussed below), transscleral CPC or MPC are non-invasive and ECP is minimally invasive.
• Severe blepharitis. If a patient has severe blepharitis, they may have a higher risk for infection with surgery, particularly trabeculectomy. Ciliary body ablation carries a lower risk of endophthalmitis.
• Risk of falling. If you’re concerned that the patient may fall and ultimately cause some damage to a bleb or cause some sort of issue with the tube, this is a potential candidate for ciliary ablation rather than a tube shunt or trabeculectomy.
• Poor follow-up. Patients who are institutionalized or in nursing homes may not be able to attend follow-up visits with regularity or use their drops as directed. Additionally, it’s difficult to account for the types of risks that may be present in their environment.
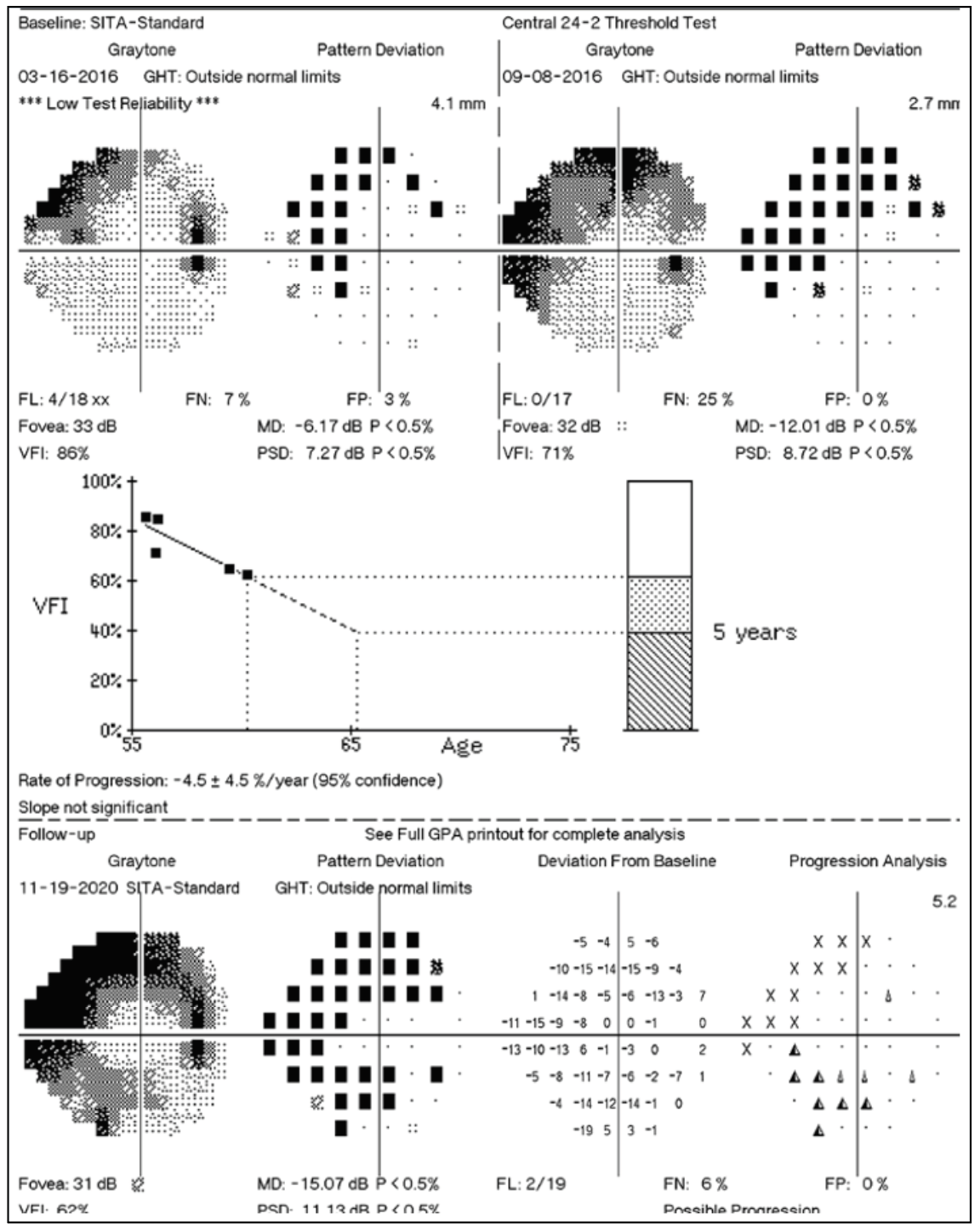 |
• Ocular tumors. When you want to avoid cutting into the eye for risk of seeding or worsening the tumor, a transscleral ciliary ablation laser procedure is a fantastic option.
• Lack of transportation. If there’s no one who can take the patient home, they may be better suited to a cycloablative procedure under local anesthesia with a retrobulbar block.
For these procedures, I’d rather avoid the greater risk of hypotony or vision loss associated with overtreating. In contrast, undertreatment may require repeat procedures.
For any patient undergoing a cycloablative procedure, be sure to counsel them that sometimes it’s not a one-time thing; that if their pressures go up later, we may need to do the procedure again.
The Options
When and how should you use CPC, micropulse CPC and ECP? Here’s a rundown of the three options:
• Traditional transscleral cyclophotocoagulation. Typically reserved for eyes with poor visual potential, CPC is an effective pressure-lowering alternative to more invasive glaucoma surgeries and can reduce IOP by 10 to 20 mmHg or more. If the outflow facility is poor, then more laser may be needed to reduce the IOP to the normal range. If extensive cycloablation is required to reach the desired IOP outcome, there may be increased risk of phthisis and vision loss.
The traditional transscleral CPC technique involves a G probe. Laser energy is applied evenly spaced around the limbus for 360 degrees, though there are some surgeons who prefer to leave one quadrant open and treat 75 percent of the way around the limbus. I aim for 20 spots total.
The conventional CPC treatment is 2,000 mW for 2,000 ms. I’ve found this to be a little less effective. In this technique, it’s generally advised to titrate up until you hear a pop and then titrate back down. However, the pops are bursting ciliary bodies and can result in inflammation, so I try to avoid this and instead use the lower and slower technique.1 The lower and slower technique uses anywhere between 1,000 to 1,500 mW for 3,000 to 4,000 ms, or four seconds per spot.
There are certain areas surgeons should avoid treating when performing this procedure, including areas with a functional trabeculectomy or tube. Areas of darker pigmentation should also be avoided. The laser applies energy that passes through the sclera and is absorbed by melanin in the pigmented ciliary body. However, if there’s pigment on the eye—such as a particularly large nevus, or there’s some blood (e.g., from an injected red eye or a small hemorrhage at the point where you cut down for a sub-Tenon’s block)—the laser energy can be absorbed and, in rare cases, even create a hole in the sclera.
Some cases may require altering the laser settings. For example, with a neovascular glaucoma patient with pressures in the 70s, much higher than your normal diode patients, performing traditional CPC at a higher setting may be suitable. Using the lower and slower technique, I’ll increase the energy from 1,000 mW to 1,500 or even 1,700 mW.
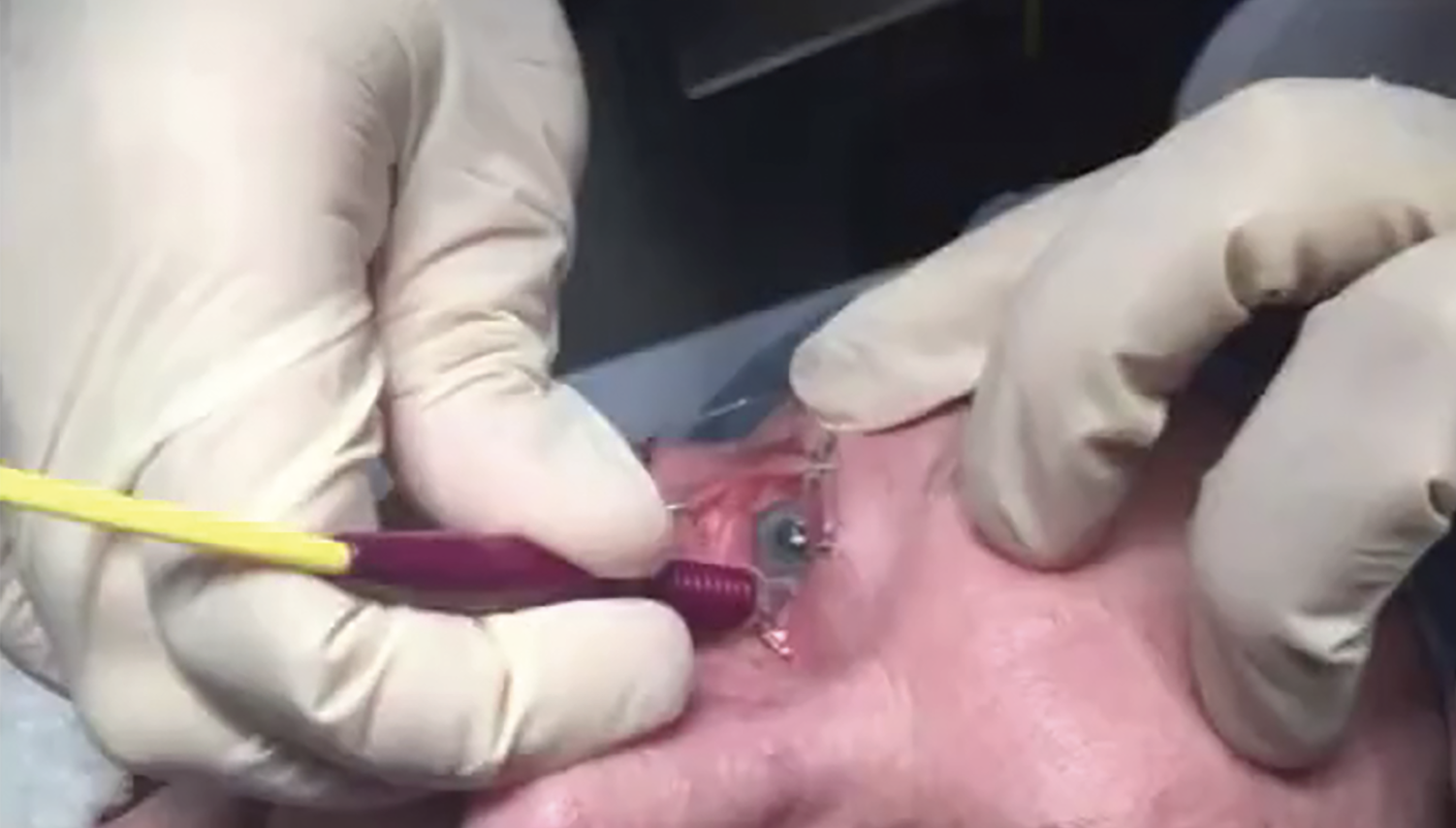 |
| Figure 2. The new micropulse P3 probe has two bunny ears which must be pointed toward the limbus in order for the probe to work properly. Photo: Ahmad Aref, MD, MBA. |
• Micropulse. Like traditional CPC, micropulse CPC is a quick procedure, taking about five to 10 minutes, with fast recovery time and no activity restrictions for patients. However, it can be used in patients with better vision. Micropulse provides a modest IOP reduction of 20 to 45 percent and lowers IOP in about four to six weeks. Patients will likely need to continue using some glaucoma medications. Micropulse is less effective in treating secondary glaucomas such as neovascular glaucoma, uveitic glaucoma as well as congenital glaucomas.
The micropulse technique is different from that of traditional CPC (Figure 2). When applying the G probe for traditional CPC in one location, it produces continuous energy. In this situation, micropulse pulses, as the name implies. It delivers the energy in the duty cycle for 31 percent of the time. This type of pulsation delivers less energy but still potentially offers adequate efficacy.
Rather than treating in discrete spots, sweep the micropulse P3 probe along the edge of the limbus. The recommended settings are 1500-2,500 mW at a 31.3-percent duty cycle. You can change this by 20 to 25 percent, if you want to increase or decrease the energy for whatever reason. A consensus panel suggested four sweeps of 20 seconds per hemisphere for a total of 160 seconds per eye (Figure 3).2 Another option is to do four sweeps of 10 to 15 seconds per quadrant.
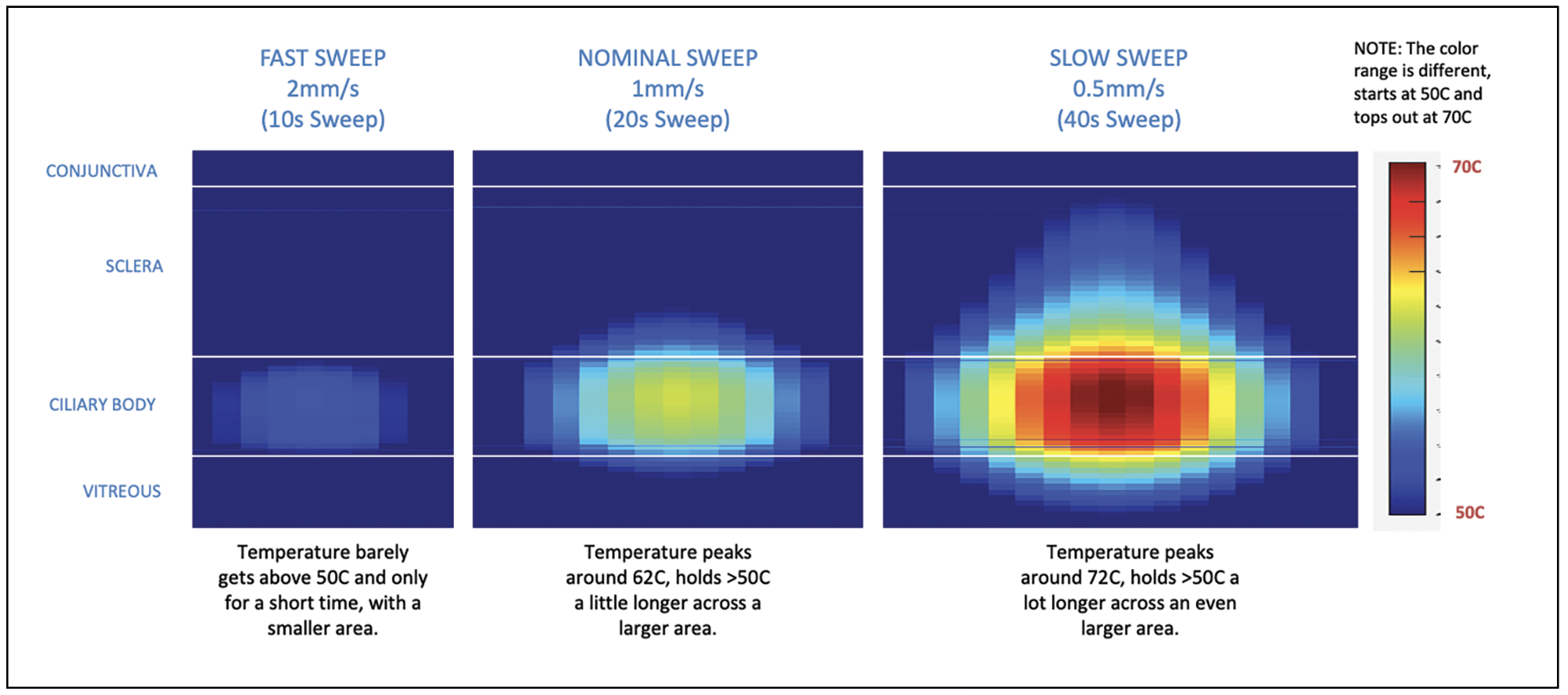 |
| Figure 3. Micropulse is more effective when performed using slower sweep speeds. Recommended settings for the micropulse technique are 2,500 mW at a 31.3-percent duty cycle, involving four sweeps of 20 seconds per hemisphere (four sweeps of 10 seconds each per quadrant). Photo: Iridex. |
A slow sweep does the trick. For comparison, a fast sweep would be a 10-second sweep, or 2 mm per second. A slow sweep is a quarter of that, let’s say 0.5 mm per second, or a 40-second sweep. Going too fast is a mistake as it reduces the amount of energy applied to the ciliary body. That may be one reason many surgeons have moved away from micropulse, not finding it to be effective. The slower you go, the greater the efficacy.
Like CPC, micropulse is an external procedure. While it can be performed in clinic, potentially with a retrobulbar block, I usually perform it in the operating room where patients can be administered propofol and perform the micropulse procedure while they’re asleep. Postoperatively, I give them a drop of atropine and that generally addresses postoperative pain.
One of the benefits of the newer cycloablative procedures—micropulse and ECP—is that the patient doesn’t necessarily need a retrobulbar block and hence wouldn’t need to be patched. For a monocular patient, this is particularly advantageous. A micropulse procedure is preferable in this scenario. A heavier micropulse may be suitable if more pressure lowering is required, and in that case, you could increase the energy a little bit or try to go even slower with the sweeps. However, I tend to use the recommended settings with the micropulse.
• Endoscopic cyclophotocoagulation. ECP may be used as a replacement for medical therapy in conjunction with other surgery (especially cataract surgery), post-trabeculectomy or post-GDD, for poor bleb candidates, in corneal transplant patients, those with congenital glaucomas and in aphakic or vitrectomized eyes, or for any indication where traditional CPC was used prior.
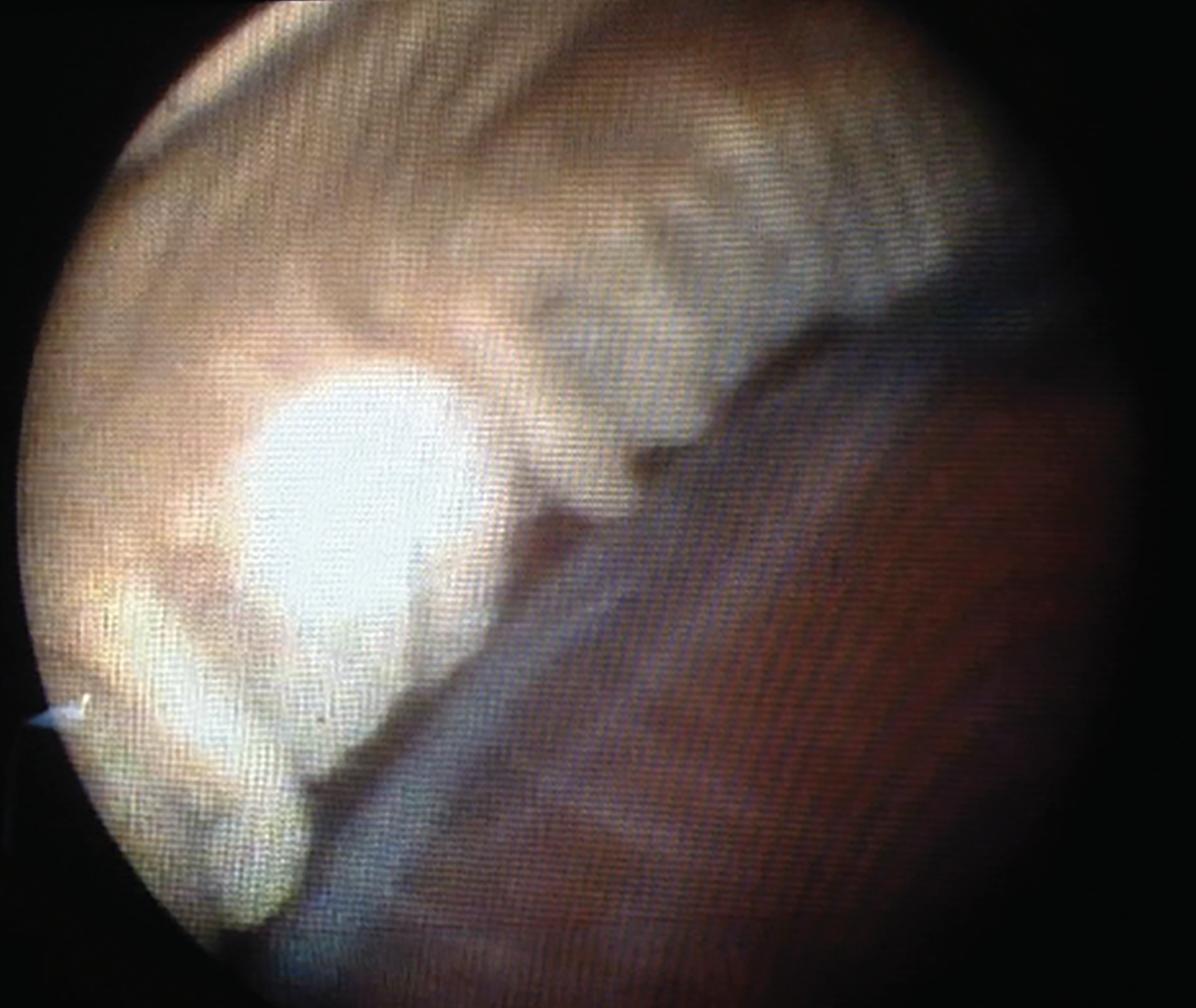 |
| Figure 4. The endpoint of ECP is whitening of the ciliary body. Note the difference in color between the ablated ciliary processes to the left and the untreated ones to the right. Photo: Malik Kahook, MD. |
ECP uses a continuous mode at 0.25 watts with a straight or curved probe. In this procedure, first make an incision, fill the sulcus with viscoelastic such as Healon. Then, insert the probe underneath the sulcus. Adjust the illumination to visualize the aiming beam. Ablate the ciliary body. The endpoint is whitening and shrinking of the ciliary processes (Figure 4). Avoid applying so much energy as to cause a pop. This causes a lot of inflammation which can be counterproductive and take longer to heal or cause edema. Ensure you have a good view and good alignment. Your view should include three ciliary processes—that’s how close you should be. Apply the laser almost as if you’re painting the ciliary processes, 270 to 360 degrees, and try to get to this endpoint of whitening.
The use of ECP as a minimally invasive glaucoma surgery employs a different approach than its use for treating an advanced glaucoma patient who’s already had a tube, for example. While I don’t use it in this context, for ECP as a MIGS procedure, many will treat ciliary body three to six clock hours, not the full 360 degrees. When performing ECP more aggressively for an advanced glaucoma patient, I’ll make two separate incisions. I usually make one incision temporally and one superiorly.
Steroid Recommendations
Cycloablative procedures can cause persistent inflammation, so you want to get that under control quickly. Some surgeons recommend using intracameral steroids at the end of the case. For traditional CPC, I’ll typically give subconjunctival dexamethasone at the time of the procedure, followed by topical steroids afterwards. For micropulse, a less intense steroid regimen may be used due to concern for steroid response. Post-procedure, I use prednisolone acetate with a fast taper, and others suggest using a weaker steroid such as loteprednol. ECP can generally be treated with intraoperative and postoperative steroids according to the surgeon’s preference, taking into consideration the need for steroid therapy required for other concomitant procedures.
Complications
Any cycloablative procedure can also cause cataract, phthisis and vision loss. Common complications with traditional CPC include hypotony, macular edema and central vision loss, with one study reporting that up to 33 percent of patients lose two lines of vision on the Snellen chart with CPC.3 This is one of the reasons CPC is reserved for worse-seeing eyes. Macular edema is a potential complication of micropulse and ECP as well, although the risk is less. It’s uncommon to have central vision loss with these procedures. Since ECP requires an incision, there’s a small albeit extremely low risk of infection. Postoperative care includes antibiotic prophylaxis against infection, in addition to steroids.
All three procedures can cause significant inflammation. ECP can even result in aggressive inflammation causing fibrin in the anterior chamber. This commonly resolves with steroids, but inflammation is a big risk with any of these cycloablative procedures.
Other Approaches
Here are a few alternative but less common ways of performing ciliary body ablation:
• Transilluminating the sclera. Transillumination involves placing a bright white light on the sclera, which will in most cases cause the ciliary body to transilluminate. You can then see the individual ciliary processes and target where you do your transscleral CPC.4
• ECP Plus. This approach targets the posterior ciliary processes, unlike regular ECP which treats the anterior portion. Typically, to access the posterior processes, you’d need to perform a vitrectomy, which most glaucoma specialists aren’t comfortable with. ECP Plus involves a limited vitrectomy followed by ECP.5 After the vitrectomy, some retina specialists sclerally depress the ciliary body and use their traditional retinal lasers on it.6
• Transpupillary ciliary ablation. In unusual situations, one or more ciliary processes may be visible at the slit lamp or on gonioscopy, such as when you’ve done a large iridectomy for a trabeculectomy or the patient has a wide dilation, is aphakic or has had a procedure whereby the iris was removed or reduced. In these cases, a transpupillary argon ciliary laser ablation can be performed in the clinic.7
In summary, ciliary body ablation has a variety of indications and can be performed in eyes that see well. Choose ECP or micropulse for eyes with better vision and earlier-stage disease and reserve traditional CPC for eyes with worse vision or end-stage glaucoma.
Dr. Saeedi is a professor of ophthalmology, vice chair of academic affairs, chief of the glaucoma division and director of clinical research at the University of Maryland School of Medicine in Baltimore, and an adjunct associate professor of bioengineering at the University of Maryland, College Park. He has no related financial disclosures.
1. Duerr ER, Sayed MS, Moster S, Holley T, Peiyao J, Vanner EA, Lee RK. Transscleral diode laser cyclophotocoagulation: A comparison of slow coagulation and standard coagulation techniques. Ophthalmol Glaucoma 2018;1:2:115-122.
2. Grippo TM, Töteberg-Harms M, Giovingo M, Francis BA, de Crom RRMPC, Jerkins B, Brubaker JW, An J, Radcliffe N, Noecker R. Evidence-based consensus guidelines series for micropulse transscleral laser therapy - surgical technique, post-operative care, expected outcomes and retreatment/enhancements. Clin Ophthalmol 2023;17:71-83.
3. Shah P, Bhakta A, Vanner EA, et al. Safety and efficacy of diode laser transscleral cyclophotocoagulation in eyes with good visual acuity. J Glaucoma 2018;27:10:874-879.
4. Agrawal P, Martin K. Ciliary body position variability in glaucoma patients assessed by scleral transillumination. Eye 2008;22;1499-1503.
5. Tan JC, Francis BA, Noecker R, Uram M, Dustin L, Chopra V. Endoscopic cyclophotocoagulation and pars plana ablation (ECP-Plus) to treat refractory glaucoma. J Glaucoma 2016;25:3:e117-22.
6. Lim JI, Lynn M, Capone A Jr, Aaberg TM Sr, Martin DF, Drews-Botsch C. Ciliary body endophotocoagulation during pars plana vitrectomy in eyes with vitreoretinal disorders and concomitant uncontrolled glaucoma. Ophthalmology 1996;103:7:1041-6.
7. Shields S, Stewart WC, Shields MB. Transpupillary argon laser cyclophotocoagulation in the treatment of glaucoma. Ophthalmic Surg 1988;19:3:171-5.




