Vitreous loss following a posterior capsule rupture is a stressful complication to encounter during cataract surgery, but keeping up to date on the appropriate approaches and anterior vitrectomy techniques can help prepare you if you end up faced with such a case. Here, surgeons review some of the risk factors and signs of a capsule rupture and discuss the best ways to respond should you lose vitreous.
Risk Factors
Preoperative knowledge is one of the best tools you can arm yourself with before a case, because certain patient factors can make a capsule predisposed to breakage. “It’s important to be aware of certain risk factors and to go into the case well aware of the individual patient’s ocular anatomy so you’re ready for any eventuality,” says Rosa Braga-Mele, MD, MEd, FRCSC, a professor of ophthalmology at the University of Toronto. “Risk factors such as posterior polar cataract are often missed because they look similar to posterior subcapsular cataract. Look for the central dot or elicit a history from the patient to see whether they were ever told they had a small cataract when they were younger. A white cataract may also be an unannounced posterior polar cataract that has progressed. When you hydrodissect in these cases, that’s often when the lens falls.”
Patients who are post-vitrectomy or post-intravitreal injection are also at higher risk for a PCR.1 Dr. Braga-Mele says that examining the posterior capsule for linear streaks in these patients’ eyes can clue you in. “Their capsule may have been brushed by the trocar or needle,” she says. “In this case, patient history may include an uneventful procedure followed by rapid development of a cataract. It’s best to wait a while before performing cataract surgery to allow the posterior capsule to scar a little more.”
Additional risk factors include very short eyes where the anatomy is more compressed, leaving you with reduced working room, and eyes with pseudoexfoliation or zonular issues, where capsular bounce or instability is more likely. “The capsule may be more likely to move and come closer to the phaco tip,” Dr. Braga-Mele says. “You could create an iatrogenic tear at that point. These challenging anatomical cases are more likely to have higher complication rates with cataract surgery.”
Better Late than Early
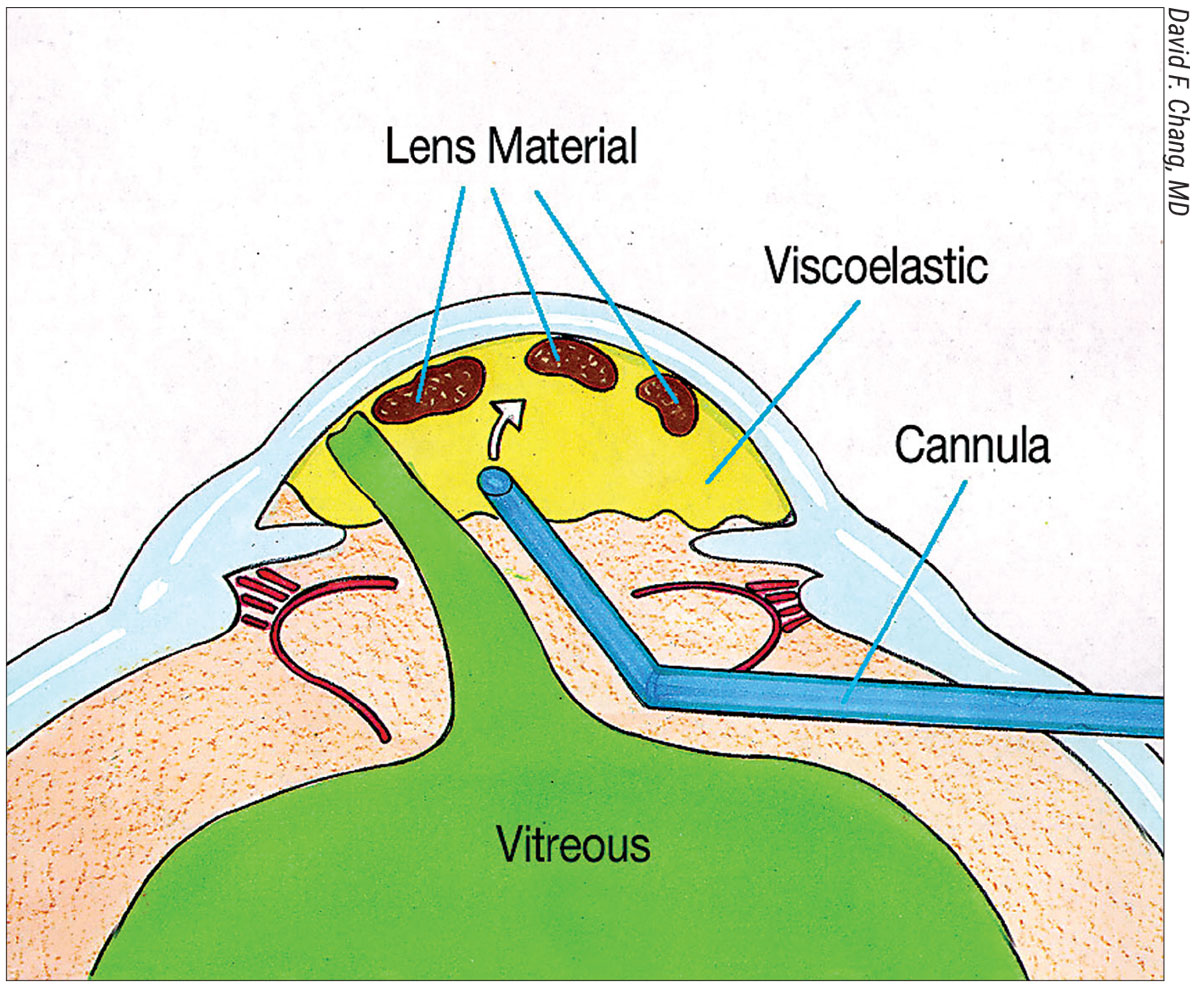 |
| Figure 1A. Injecting a dispersive OVD (yellow) maneuvers lens fragments anteriorly and peripherally prior to filling the AC. |
Some cases of PCR are unavoidable, but you can take steps to reduce the risk by performing good hydrodissection, says Steve Charles, MD, founder of the Charles Retina Institute in Germantown, Tennessee, and clinical professor of ophthalmology at the University of Tennessee. “Make sure the lens rotates in bag. I use Alcon’s Ozil handpiece and perform careful irrigation/aspiration.”
Dr. Braga-Mele notes that for cases where the patient may be predisposed to PCR, hydrodissection isn’t always the best choice. “I don’t hydrodissect in cases where I fear for the integrity of the posterior capsule,” she says. “If you know the case is high-risk, use viscoelastic to your advantage. I viscodissect, viscodelineate or hydrodelineate to remove the nucleus and then deal with the epinucleus and cortex.
“If you have a case where PCR may be inevitable due to the eye’s anatomy, it’s better that the PCR occur as late as possible rather than earlier in the procedure when the nucleus can drop,” she emphasizes. “With an earlier PCR, you’re more likely to have nuclear fragments go into the vitreous.” She advises using a dispersive rather than a cohesive viscoelastic in these cases because it creates space more effectively.
In the Hot Seat
Posterior capsule rupture and vitreous loss are rare occurrences in cataract surgeons’ careers. The incidence of PCR during phacoemulsification among experienced surgeons ranges from 0.45 to 3.6 percent.2 Among surgeons recently converting from extracapsular cataract extraction to phacoemulsification, the rates are reported to be 4.8 to 11 percent.3 Dr. Braga-Mele says that the comments she hears most often from experienced and high-volume cataract surgeons are, “I don’t remember what my settings should be,” or “I don’t know how to do this because I haven’t done one in about five thousand cases.”
“Refresher articles and courses at the Academy and ASCRS are good for us to do every once in a while to keep up to date on what we should be doing if something should happen,” Dr. Braga-Mele says. “If you perform a good vitrectomy, clean-up and lens implantation, your patient can have an outcome as if no complication occurred.”
If you encounter a sudden rupture, here are a few things to keep in mind:
• First, recognize that a posterior capsule rupture has occurred. Surgeons note that residents in particular sometimes fail to notice a posterior capsule rupture. Missing this event could result in retinal tears and detachments from vitreous traction. “If you see sudden deepening of the anterior chamber or capsule wrinkling, be on your guard,” says Dr. Charles. Other signs include pupil snap sign, difficulty rotating a previously mobile nucleus (due to vitreous in the capsular bag), excess sideways tilt of the lens or IOL, moments where the pupillary margin is inconsistent with anterior chamber manipulation, and strands of vitreous in the anterior chamber.
Of course, experience helps. In a study reviewing 14,520 cataract surgeries performed at a single center in Finland spanning eight years, researchers confirmed that practice makes for fewer complications. They identified 144 cases (0.99 percent; mean age 76.9) of posterior capsule rupture and/or loss of capsular bag support.4 Capsular bag-related complications occurred in 0.36 percent of surgeries performed by senior surgeons and in 7.03 percent of surgeries done by residents. Toward the end of the study, these incidence rates improved for residents (1.32 percent) and remained about the same for senior surgeons (0.32 percent). The gradual decline in complications pointed to increasing surgical experience among both resident and senior surgeons.
Another study from 2013 (n=500 eyes) of resident-performed cases reported that 51 eyes (10.2 percent) developed vitreous loss and 48 eyes (9.6 percent) developed posterior capsule rupture and vitreous loss. Risk factors included diabetes, shallow anterior chamber, absence of supervision, larger capsulorhexis, anterior capsule tear and longer effective phaco time. The researchers concluded that direct attending supervision and careful case selection for the level of cataract surgery residency is necessary for avoiding sight-threatening complications.5
• Your initial reaction is key. It’s important not to panic and withdraw your instruments. “If lens material falls into the vitreous cavity, pause first,” says Dr. Charles. These fragments will remain supported by the vitreous and won’t damage the retina, but fishing for them could.
• Avoid rupturing the anterior hyaloid face. When you recognize that a PCR has occurred, your first objective is to avoid rupturing the anterior hyaloid face, says David F. Chang, MD, of Los Altos, California, and clinical professor of ophthalmology at the University of California San Francisco. “The sudden anterior chamber decompression caused by abruptly withdrawing the phaco tip will cause vitreous to prolapse forward toward and through the phaco incision,” he says. “This will expand the posterior capsule opening and set the stage for a cascade of problems.”
• Assess the extent of the capsule rent. “If there’s a small rent in the capsule and no vitreous in the anterior segment, perform a posterior continuous capsulorhexis to contain the rent and prevent it from extending,” says Dr. Braga-Mele. “If the rent is larger, then the key is to remove all of the nuclear fragments before you start to lose vitreous.”
• Use a generous amount of OVD. If you’ve already lost vitreous, Dr. Braga-Mele says to push the vitreous back with dispersive viscoelastic. “I then pull any nuclear fragments into the anterior segment over the iris, using the iris as the scaffold to hold the nuclear segment,” she explains. “You might even put in a Sheet’s glide to partition the vitreous away from the anterior segment and hold the vitreous and the posterior capsule tear back. If there’s no vitreous in the anterior segment, I use a dispersive viscoelastic as my second instrument so I can keep pushing vitreous back as I remove the nuclear segment.”
• Avoid cellulose sponges and impaling the residual nucleus with the phaco tip. “Mopping externally prolapsed vitreous with a Weck-Cel sponge causes traction on the vitreous base,” Dr. Chang says. “Additionally, you shouldn’t attempt to impale the residual nucleus with the phaco tip. This risks vitreous incarceration and a giant retinal tear. Because residual nuclear fragments are supported by the vitreous, they’ll likely descend posteriorly as an anterior vitrectomy is performed. Injecting OVD via a side port paracentesis prior to withdrawing the phaco or IA tip is key to preventing forward vitreous prolapse and rupture of the hyaloid face.”
Performing Anterior Vitrectomy
If there’s vitreous in the anterior chamber, it’s time to perform an anterior vitrectomy. But before beginning, it’s important to ensure the patient’s pupil dilates and that the cornea is reasonably clear (i.e., no extensive miosis or keratopathy). Experts say your goals for this procedure should be to minimize both intraoperative and postoperative vitreoretinal traction, as well as any mechanical trauma to the iris, which is a major cause of CME.
“Once vitreous prolapse has been identified, the foremost objective is to avoid aspirating vitreous with the phaco or IA tip,” says Dr. Chang. “Never attempt to spear or aspirate residual nucleus with the phaco tip, despite the temptation to prevent its descent. Once the posterior capsule is open, it’s the vitreous that keeps nuclear material from descending onto the retina.”
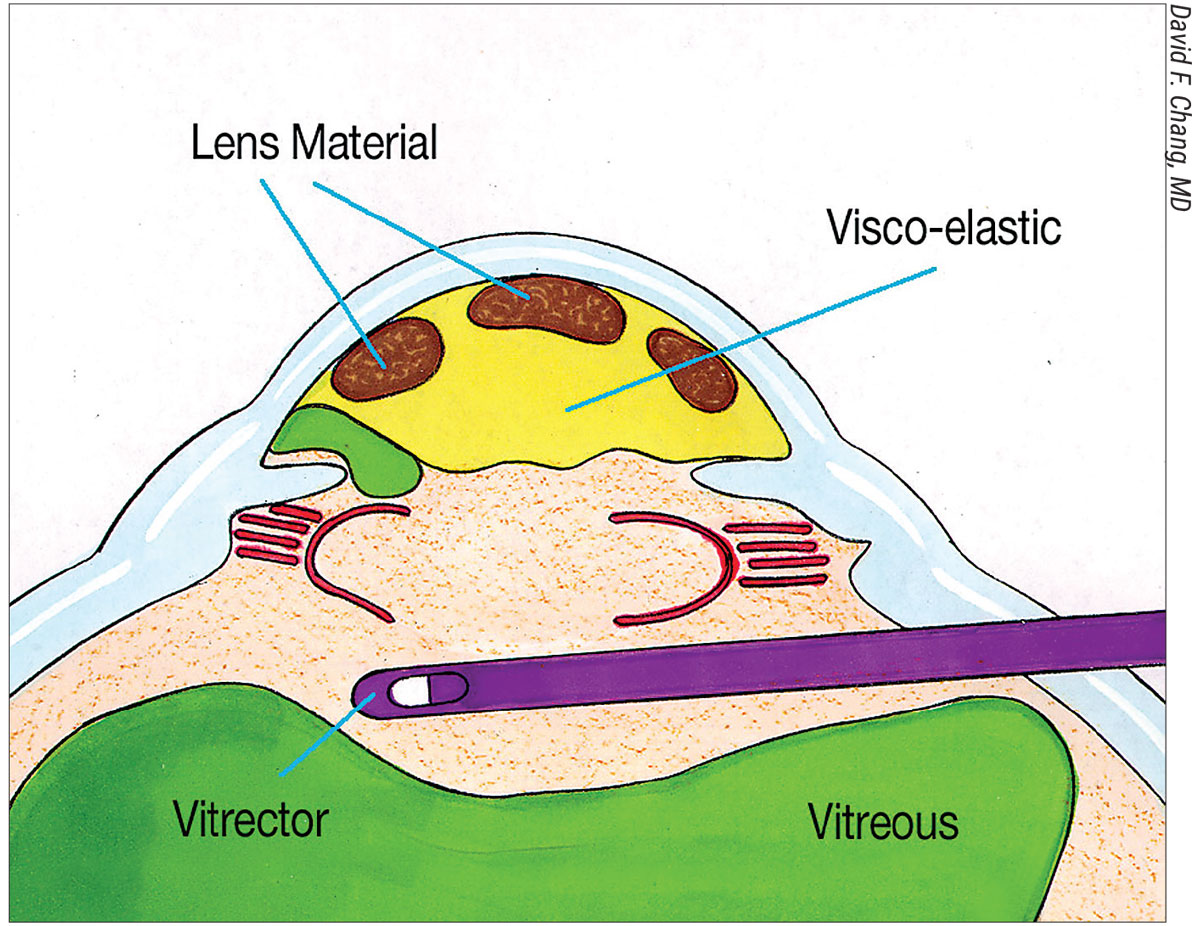 |
| Figure 1B. The vitrectomy cutter is introduced via a pars plana sclerotomy and transects any forward prolapsing bands of vitreous (green), while remaining behind the pupil. This preserves the dispersive OVD in the AC. |
He advocates a sequence of steps that he calls the “Viscoelastic Trap.” To perform it, first inject triamcinolone into the AC to stain any prolapsing vitreous. Next, use a dispersive OVD to maneuver lens material anteriorly and peripherally above the iris (Figure 1A). Then, completely fill the AC with dispersive OVD. “I then perform a biaxial anterior vitrectomy through a pars plana sclerotomy, with a self-retaining infusion cannula placed through a limbal paracentesis (Figure 1B).
“The goal is to keep the vitrectomy tip behind the pupil while excising the central anterior vitreous and transecting any forward-prolapsing vitreous bands,” he continues. “In this way, the dispersive OVD filling the AC is not removed; it remains in place, continuing to trap all the loose lens material. Once the bands of prolapsed vitreous have been cut, it’s safe to remove the remaining OVD-suspended lens material. This might be with biaxial IA for epinucleus and cortex, or with a phaco tip for nuclear fragments. For the latter, place a three-piece IOL in the sulcus or into the AC to serve as an IOL scaffold. Popularized by Amar Agarwal, the IOL optic prevents nuclear fragments from descending while simultaneously blocking vitreous from being aspirated. Any loose bits of vitreous in the AC that have been transected from their attachment to the vitreous base can and will be safely aspirated in the process.”
Dr. Braga-Mele says that one of the keys to removing vitreous is to perform the anterior vitrectomy bimanually, splitting irrigation and aspiration by creating two new wounds. “You have to go to a bimanual vitrectomy system at this point and abandon your main wound if you have vitreous,” she says. “Clean up the vitreous and push it back. When you’re cutting or removing vitreous, it’s important to do irrigation, cut then aspiration, as opposed to when you phaco, where you do irrigation, aspiration, then cut. You want to be sure you’re never pulling on vitreous, but always cutting it to avoid retinal traction.
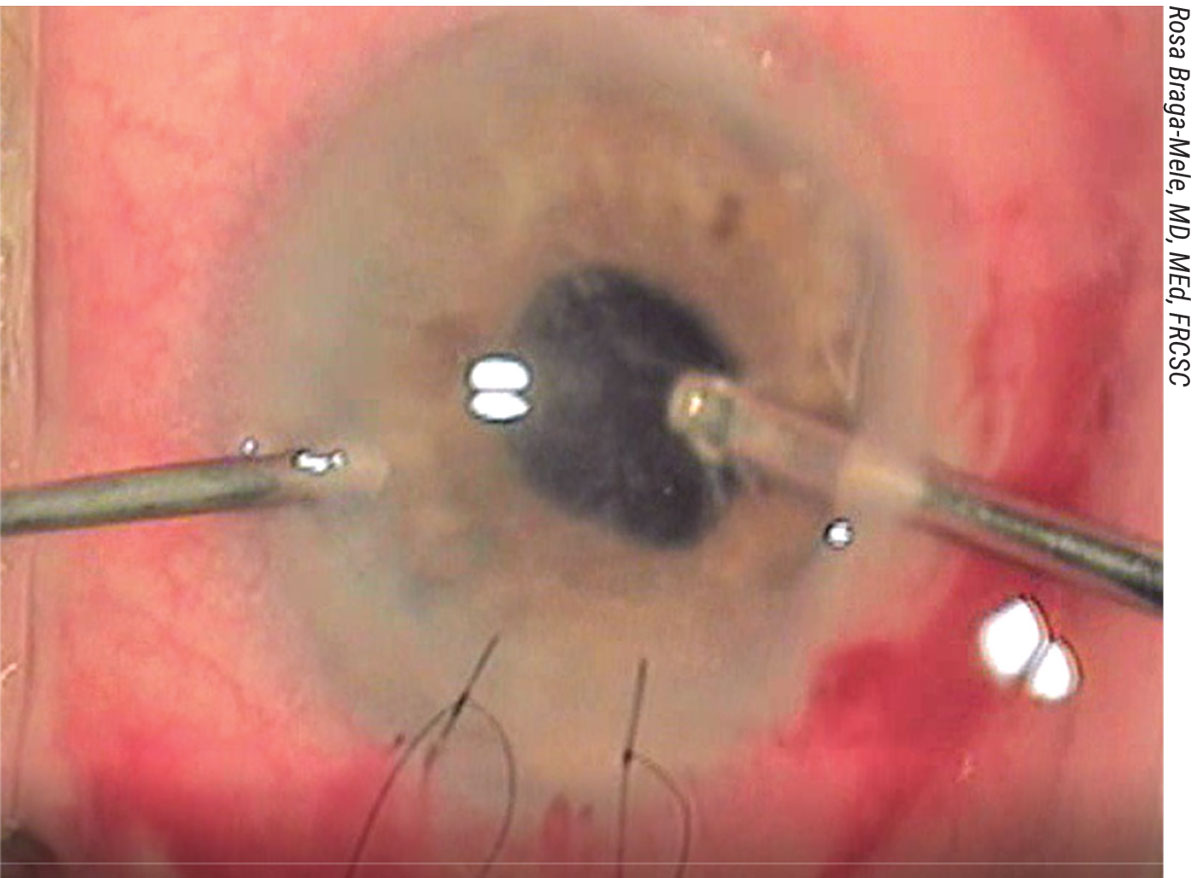 |
| Figure 2. Intraoperative image showing a bimanual anterior vitrectomy with the main incision sutured and the vitreous stained with triamcinolone peripherally prior to filling the AC. |
“The reason you abandon your main wound is that vitreous will go to the area of least resistance, and the main wound is that place,” she says. “If you go through the main wound you’ll just keep expressing more vitreous.”
Dr. Charles adds that you should never withdraw the vitreous cutter while vacuum is applied. “You can cause retinal breaks if the cutter is withdrawn while vacuum is applied,” he says. “Excessive flow rates or less-than-maximum cutting rates can also cause traction. For your parameters, you should use the highest available cutting rate and very low infusion pressure/bottle height, as well as the lowest effective flow rate.” The cut rate should be at least 1,200 cpm or more.
Another key to the anterior vitrectomy is clearing away all of the vitreous before continuing to remove any nuclear fragments. One way, considered old-fashioned and no longer advised because it results in retinal traction, is the use of cellulose sponges to test for vitreous. “For this method, typically, if the pupil is round and there aren’t any strands coming to the Weck-Cel site, then you’ve removed enough vitreous to keep moving forward,” says Dr. Braga-Mele.
“The other, preferred way to check for residual vitreous is by using intracameral triamcinolone,” she continues. “It’s available without preservatives from certain companies. Triamcinolone will stain the surface of the vitreous, but you must keep reinserting it because it only stains the surface. Proceed and cut away what you see and then reinsert a bit more to see if you got it all. Use Miochol-E (acetylcholine chloride intraocular solution; Bausch + Lomb) and Miostat (carbachol 0.01% intraocular solution; Alcon) to shrink the pupil. If the pupil comes down into a really nice, tight 2- to 3-mm pupil, then you know you’ve probably removed most of the vitreous, because vitreous will cause pupil peaking.”
If you’re not careful, you can vitrectomize the capsule and wind up with nowhere to put the IOL, she adds. “Also, removing too much vitreous can cause the eye to become over-decompressed or cause choroidal edema.”
Limbus or Pars Plana
Should anterior segment surgeons perform a pars plana vitrectomy during cases such as these? This question has generated a lot of debate. “Pars plana vitrectomy is better if the surgeon is trained,” says Dr. Charles. “It’s better for the corneal endothelium and the iris. Always infuse through a sideport, with the cutter through the pars plana or second sideport, bimanually.”
However, many anterior segment surgeons aren’t comfortable or experienced with performing an anterior vitrectomy through a pars plana sclerotomy, Dr. Chang points out. “Performing a biaxial limbal vitrectomy may be the more comfortable and familiar alternative,” he says. “Any posteriorly retained lens material can be subsequently removed by a vitreoretinal surgeon, with an excellent prognosis. Regardless of the vitrectomy approach, repeated triamcinolone injections can confirm complete excision of any prolapsed vitreous.”
Dr. Braga-Mele agrees, saying that cataract surgeon comfort should be the determining factor. “The main thing is to split irrigation and aspiration, whether you’re performing an anterior limbal or modified posterior vitrectomy,” she says. “If you’re doing a posterior vitrectomy, you must understand the anatomy of choroidal vessels and not go into the three or nine o’clock positions, because you could cause choroidal hemorrhage. Go only 3.5 mm posterior from the limbus to avoid causing occult tears.
“I still use my irrigation anteriorly through the limbus, even when I use a vitrector posteriorly,” she continues. “You don’t need to do a conjunctival cut-down when you do a posterior vitrectomy, but you should use a clean MVR or sideport blade to make the incision so you don’t traumatize the retinal anatomy. Lastly, you need to cut on the way out and make sure no vitreous comes back out through the posterior vitrectomy incision.”
Lens Implant Options
Dr. Chang says the remaining capsular anatomy will determine the IOL options available to you. “With a large posterior capsular rent but an intact capsulorhexis, a three-piece IOL in the sulcus with optic-CCC capture is an excellent strategy,” he says. “If there’s a wraparound anterior capsular tear that extends across the posterior capsule, I would consider a three-piece IOL in the sulcus without optic-CCC capture.
“Options for implanting a single-piece acrylic refractive IOL (e.g., toric or EDOF) might include converting a PC defect into a posterior CCC, or using the anterior CCC for reverse optic capture,” he adds. “If there’s insufficient capsular support, scleral fixation of an IOL is a consideration. A properly sized and positioned AC IOL is another acceptable option, particularly if the surgeon isn’t comfortable with alternative techniques of non-capsular IOL fixation.”
Dr. Braga-Mele notes that if she performs a posterior vitrectomy, she pairs herself with a retinal surgeon. “This way, the retinal specialist can perform a good retinal exam for me,” she says. “You can induce a giant retinal tear if you’re not careful.” She has patients see a retinal specialist two to six weeks after surgery.
Dr. Charles is a consultant for Alcon. Dr. Braga-Mele is a consultant to Alcon, LensGen and Zeiss. Dr. Chang reports no relevant financial disclosures.
1. Miller DC, Christopher KL, Patnaik JL, et al. Posterior capsule rupture during cataract surgery in eyes receiving intravitreal anti-VEGF injections. Curr Eye Res 2021;46:2: 179-184.
2. Vajpayee RB, Sharma N, Dada T, et al. Management of posterior capsule tears. Surv Ophthalmol 2001;45:473-88.
3. Narendran N, Jaycock P, Johnston RL, et al. The Cataract National Dataset electronic multicenter audit of 55,567 operations: A risk stratification of posterior capsule rupture and vitreous loss. Eye (Lond) 2009;23:31-7.
4. Aaronson A, Viljanen A, Kanclerz P, et al. Cataract complications study: An analysis of adverse effects among 14,520 eyes in relation to surgical experience. Ann Transl Med 2020;8:22:1541.
5. Hashemi H, Mohammadpour M, Jabbarvand M, et al. Incidence of and risk factors for vitreous loss in resident-performed phacoemulsification surgery. J Cataract Refract Surg 2013;39:9:1377-82.