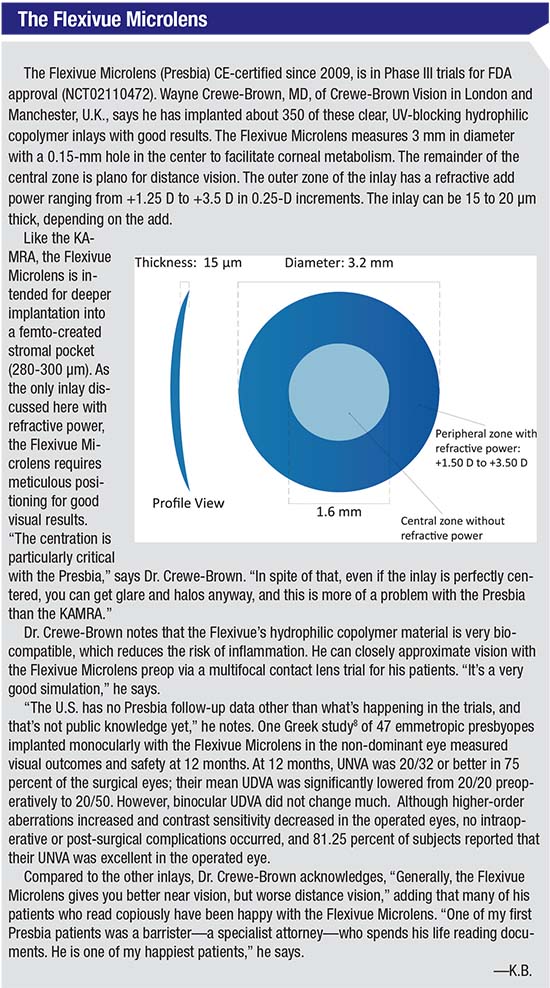
The KAMRA (AcuFocus), the Raindrop (ReVision Optics), and the Presbia Flexivue Microlens (Presbia) each treat presbyopia via different mechanisms. The KAMRA is a donut-shaped inlay made of dark polyvinylidene fluoride, 3.8 mm in diameter with a 1.6-mm aperture and 8,400 microperforations that permit nutrients and oxygen to reach the cornea. The KAMRA relies on the pinhole effect to enhance near vision, allowing only central rays of light onto the cornea to enhance depth of focus. Approved by the FDA in 2015, the KAMRA is meant for monocular implantation in the non-dominant eye, into a femto-created stromal pocket at a minimum depth of 200 µm. Wayne Crewe-Brown, MD, of Crewe-Brown Vision Centers in London and Manchester, U.K., has approximately 1,000 KAMRA cases and some 350 cases with the Presbia Flexivue Microlens to his credit.
The Raindrop, FDA approved in 2016, is a biocompatible hydrogel inlay that measures 2 mm in diameter and 32 μm thick at the center, tapering to 10 μm thick at the periphery. Consisting of 77 percent water and devoid of inherent refractive power, the Raindrop steepens the central cornea when implanted in the non-dominant eye under a femto-made flap at the manufacturer-recommended depth of at least 150 μm and 30 percent of central corneal thickness. Jeffrey Whitman, MD, chief surgeon and founder of Key Whitman Eye Centers, based in Dallas, has more than 200 Raindrop procedures to his credit, and was an investigator in the FDA clinical trials.
Wound-Healing Issues
By definition, the inlay is a foreign body inside the eye. In a small number of patients, the body’s protective inflammatory response goes into overdrive, creating haze and fibrosis local to the inlay. In the early stages of KAMRA’s development, it was implanted under a flap until this wound-healing response was noted. “I spent very little time doing that with KAMRA before switching to a pocket, and I probably would not have carried on with the KAMRA if we hadn’t developed a pocket technique,” says Dr. Crewe-Brown, adding that he also considered the flap technique to be associated with unacceptable dry-eye risk.
Dr. Crewe-Brown says that switching to a stromal pocket has helped to greatly reduce the number of KAMRA patients requiring explantation due to overly aggressive wound-healing responses. “It’s not a big deal anymore, but it’s something we still see from time to time,” he says. On those occasions that a strong wound-healing response still occurs, Dr. Crewe-Brown chooses his words carefully when discussing it with patients. “We call it ‘wound healing,’ because if you use the word ‘inflammation’—which it is, as well—the patient immediately thinks it’s an infection,” he says. “It’s much less common now in the pocket than when they were under a thick flap.
“You can
 |
Dr. Whitman also notes fibrosis as a rare complication in Raindrop procedures. “Some patients are going to have a reaction to having something under a flap, and we don’t have a way of knowing beforehand who’s at risk,” he says. Although he emphasizes that fibrosis is rare, Dr. Whitman cautions that any sudden and dramatic postoperative improvement in near vision is actually an ominous sign. “One of the things that I warn other surgeons about is that when patients get fibrosis, they tend to get better reading vision because they get further steepening of the cornea,” he explains. “I’ll tell them that’s a bad sign, and that they have to tell their patients it’s a bad sign. If they see their near vision getting better and their distance vision getting worse, it may be that they’re developing some fibrosis, and they need to come in and get it treated. If we treat it early, we can usually get rid of it with steroids.
“What may eventually happen is that there will be so much haze that their vision will go bad,” Dr. Whitman continues. “If things get hazed up far enough, it’s a foreign-body reaction so severe that they could get a melt in the cornea,” he warns. In the FDA trial, Dr. Whitman did observe some fibrosis, but none in his private practice with Raindrop to date. The trial followed 373 patients with Raindrop in the non-dominant eye for a year. Of the 340 eyes evaluated at one year, 14 percent experienced haze, which responded to steroids in all but one case. Eleven eyes were explanted for causes including haze, surgical complications and patient dissatisfaction.1
Dr. Whitman believes that his intraoperative use of mitomycin-C has prevented fibrosis in his patients to date. He credits Julian Theng, FRCOphth, M.Med, FRCSEd, with adding mitomycin-C to the Raindrop procedure. Dr. Theng presented a paper on combining LASIK, mitomycin-C and Raindrop implantation at the American Society of Cataract and Refractive Surgeons 2017 Symposium and Congress in Los Angeles. “We do a flap, LASIK, and mitomycin-C off label, and then put the inlay in,” says Dr. Whitman. “We see nice clear flaps and, so far, very nice, clear inlays using that regimen.” Dr. Whitman is currently participating in a three-center clinical trial of mitomycin-C in Raindrop procedures (ClinicalTrials.gov Identifier: NCT03101501).
Infection
“As far as I know there’s never been an infection reported, even in the FDA trial,” says Dr. Whitman of the Raindrop. “But if you think about the number of infections that occur with LASIK, they’re almost nonexistent in this day and age. Never say never, but it would be very, very rare.”
Dr. Whitman and
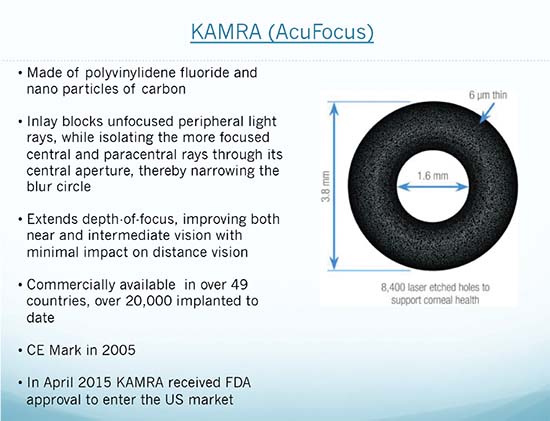 |
| The latest KAMRA model is 6 μm thick and designed for implantation into a stromal pocket at least 200 μm deep, potentially avoiding side effects that might accompany shallower placement under a flap. |
In a case series of five eyes in Ireland (three implanted with KAMRA; two with the Flexivue Microlens) with suspected infectious keratitis from six days to four months postop (one case confirmed by culture; all responded favorably to antibiotics), two of the KAMRAs were explanted, but all eyes had residual visual acuity decreases due to stromal scarring.2
Epithelial Ingrowth
Although Dr. Crewe-Brown has not seen epithelial cell ingrowth affecting the stromal pockets for either KAMRA or Presbia Microlens, he takes preventative measures to discourage it. “We train surgeons that when they put the inlay in the pocket, they have to lift up the edge of the pocket with a flap lifter or something to make sure they don’t catch any cells while pushing the inlay into the pocket,” he explains. “You’ve got to be aware of it, but in my thousand cases of KAMRA and 350 with Presbia, I’ve never had pocket epithelial ingrowth.” He says that besides lifting the pocket edge, another basic tenet of his surgical technique likely prevents ingrowth. “I use a fair amount of fluid, so I think I wash out a fair number of cells that might otherwise wind up in the pocket,” he says. A five-year study of 32 eyes implanted with an older model of the KAMRA (ACI7000, which was thicker and had fewer perforations) under a flap showed one case of epithelial ingrowth.3 In U.S. clinical trials of the latest version (ACI7000PDT) of the KAMRA, the incidence of epithelial ingrowth was 0.6 percent.4
Dr. Whitman didn’t see epithelial ingrowth in his clinical trial patients, although it was reported in 11/373 eyes at 36 months,1 and he hasn’t encountered it in his commercial patients. “There’s no higher risk to this than doing a LASIK flap, but it can happen,” he acknowledges. “I really think that since we’ve gone to femtosecond lasers the incidence of epithelial ingrowth has come way down. With microkeratomes, it used to be more common because the seal wasn’t as good. Now, because we can conform the geometry better for a better seal when we put the flap back down, epithelial ingrowth is thankfully very rare.”
Visual Issues
Possible causes of postoperative vision problems include inlay decentration, dryness and poor neuroadaptation. Decentration of the Raindrop in clinical trials occurred in 18/373 eyes, which required replacement of the inlay.1 With experience, Dr. Whitman has found a way to improve the efficacy of repositioning the Raindrop when it becomes necessary. “We’ve had two that slipped inferiorly in the first week,” he says. “We took them out for about three weeks, then we put them back in.” This technique differs from the protocol during FDA investigations, where the investigators moved decentered inlays back into place in a single procedure. “I found that if you just moved them back into place and put the flap back down, the very next day, they kind of followed the indentation or track of where they had decentered to in the first place. The inlay would go right back to the same place. Now, I take it out and put it back in later; now it’s not a problem,” he explains.
Recentration of a malpositioned KAMRA was associated with improved UNVA in a small study conducted right after FDA approval.5 The same study found that stromal pockets ≤ 250 μm deep were associated with better UNVA. “You’ve got to be very careful about centration,” Dr. Crewe-Brown says. “You must also be very careful that you don’t explant an inlay because of glare and halos that could’ve been fixed by re-centering it. I’ve certainly, in my time, re-centered a fair number of both of the inlays [KAMRA and Presbia]. But as I get better and better at centering them during the primary surgery, that doesn’t happen much.”
A more common factor in poor visual outcomes with inlays is dry eye. Of Raindrop patients in the clinical trial, 93 percent achieved UNVA of 20/25 or better, and 95 percent of them reported no or mild dry eye.1 In the KAMRA trial, 5.8 percent complained of severe dry eye at 12 months, dropping to 5.5 percent at 36 months.6 “Dry eye is the enemy of vision with inlays,” says Dr. Whitman. “Anytime you change the curvature of the eye to give more than one level of vision, dryness is the enemy.” He recommends standard dry-eye treatments like punctal plugs and drops, adding that postop dry eye usually improves after severed nerves into the surface of the cornea grow back into the flap.
“Doctors don’t like failure; neither do patients,” says Dr. Whitman, who nonetheless prepares his Raindrop candidates for the possibility that they may be among the few patients unable to tolerate a non-dominant eye programmed to see differently than their fellow eye. ‘Most patients tend to neuroadapt over three months,” he says. “If they aren’t doing better by three or four months and are unhappy, we’ll lift the flap and remove it. Anytime patients are unhappy I’ll say, ‘Some people take three months before things kick in, but if you’re unhappy, we’ll have to consider that maybe this is not for you.’ But thankfully, that’s very rare.” He reports that only one of his patients to date has requested removal because he couldn’t tolerate the visual discrepancy between his eyes.
“Every patient is told up front that about 2 percent of patients will need the inlay to be explanted,” says Dr. Crewe-Brown. “That’s not the doctor’s fault; it’s not the patient’s fault. It’s just the problem certain patients have coming to terms with having different vision in each eye. That’s where there is an overlap with monovision. A lot of cynical doctors say that inlays are nothing more than monovision. That’s not correct, because inlays do give better distance vision in the reading eye than monovision. But your brain still has to get used to the fact that through one eye, your vision is slightly different than the other,” he says.
Dr. Whitman says that preop contact lens trials were done for Raindrop patients in the FDA trials to
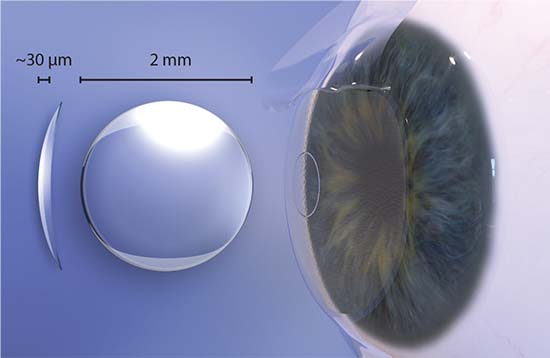 |
| The Raindrop’s manufacturer says that this tiny, clear hydrogel inlay is made of highly biocompatible materials similar to those used to make soft contacts. Implanted under a stromal flap at 30 percent central corneal thickness, it enhances near vision by creating a hyperprolate cornea. |
All of Dr. Crewe-Brown’s Flexivue Microlens patients get a multifocal contact lens trial, since that inlay has refractive power and patients will get a closer approximation of the postop discrepancy between their eyes. However, he currently doesn’t have access to an appropriate trial contact lens for KAMRA. “But there’s talk of a Chinese company producing a pinhole contact lens, which I’m looking forward to, because I’m hoping to be able to use that as a simulator,” he says.
Easily Reversible
Both surgeons find corneal inlays’ additive nature and ease of reversibility reassuring in the event that things don’t go to plan. “One of the beauties of this versus almost every other technology I use is that it’s easy to undo,” observes Dr. Whitman. “I can give inlay patients back their original vision.”
Dr. Crewe-Brown will explant a KAMRA or Presbia under certain circumstances. “I tell all patients to allow six months for their adaptation process. Unless there is an infection or the patient is violently unhappy, I will not remove the inlay before six months. It’s a big adaptation, and patients need to be given time,” he says. If a patient ultimately needs an inlay removed, he says the procedure is not nearly as problematic as an IOL removal and exchange can be. “Remove a multifocal IOL, and you’ve got a difficult operation to get it out without causing damage, and then you’ve got to put a monofocal intraocular lens into the eye, and the patient’s going to end up with only distance vision—which is not why they came to you in the first place,” he says. “But I can give an inlay patient back their cornea pretty much as it was before the inlay. That, to me, is one of the big distinctions between the two ways of treating presbyopia.”
A 2015 review of published studies on KAMRA, Raindrop, and Flexivue inlays said of reported complications: “None of the published studies on these corneal inlays published data on serious or sight-threatening complications. Only a few cases of epithelial ingrowth and complaints of glare, halo, dry eye, or night vision problems were named. Ingrowth was either resolved in all cases and/or did not affect the visual axis. The complaints were described by patients as being mostly mild to moderate, and they happened to be the same complications as those encountered after LASIK.”7
The general sense of inlays as low-risk may shift as more long-term follow-up becomes available. But right now, in some patients at least, the available and emerging corneal inlays may represent the new standard of care for corneal-based surgical presbyopia correction, ostensibly offering improved near vision without sacrificing distance vision—or ocular health. “We all like to feel better, different or younger for some reason. There’s a good feeling about waking up in the morning and being able to see the alarm clock or read the back of the shampoo bottle,” Dr. Whitman observes. “We’ve jumped through a lot of hoops to get inlays to a point where they are very good,” says Dr. Crewe-Brown. “But who’s to say they’re not going to get better? That’s why I hang in there. That’s why I do them. That’s why we have patients who want them.” REVIEW
Dr. Crewe-Browne is a member of the medical advisory boards of AcuFocus and Presbia. Not a paid consultant, he is awarded travel expenses by both companies. Dr. Whitman is consultant to ReVision Optics.
1. Whitman J, Dougherty PJ, Parkhurst, GD, Olkowski J, Slade SG, et al. Treatment of presbyopia in emmetropes using a shape-changing corneal inlay: One-year clinical outcomes. Ophthalmology 2016;123:3:466-75.
2. Duignan ES, Farrell S, Treacy MP, et al. Corneal inlay implantation complicated by infectious keratitis. Br J Ophthalmol 2016;100:2:269-73.
3. Dexl AK, Jell G, Strohmaier C, et al. Long-term outcomes after monocular corneal inlay implantation for the surgical compensation of presbyopia. J Cataract Refract Surg 2015;41:3:566–75.
4. Vukich JA, Durrie DS, Pepose JS, Machat MD et al. Addressing Presbyopia with the KAMRA Inlay. AcuFocus company communication.
5. Moshirfar M, Quist TS, Skanchy DF, Wallace RT, Linn SH, Hoopes PC Jr. Six-month visual outcomes for the correction of presbyopia using a small-aperture corneal inlay: single-site experience. Clin Ophthalmol 2016; 10: 2191–2198.
6. Data on file, AcuFocus, Inc.
7. Arlt EM, Krall EM, Moussa S, Grabner G, Dexl AK. Implantable inlay devices for presbyopia: The evidence to date. Clin Ophthalmol 2015;9:129-37.
8. Limnopoulou AN, Bouzoukis DI, Kymionis GD, et al. Visual outcomes and safety of a refratctive corneal inlay for presbyopia using femtosecond laser. J Refract Surg 2013;29:1:12-18.



