A 28-day, double-masked, multicenter Phase-III study of the steroid Durezol (difluprednate ophthalmic emulsion) sponsored by the drug's developer Sirion Therapeutics recently found the drug to have efficacy similar to Pred Forte in 90 patients with acute anterior uveitis.
In the study, 50 patients were randomized to receive Durezol q.i.d. and 40 to receive Pred Forte eight times a day for an initial period of two weeks. At day 14, the Durezol patients showed a mean cell grade reduction of 2.1 and the Pred Forte group had a mean grade of 1.9, which confirmed the study's endpoint of non-inferiority of Durezol compared to Pred Forte. The drugs were tapered over the remaining weeks, and, if any patient got worse at any point, he was removed from the study for more intensive treatment.
Thomas Flynn, MD, assistant professor of ophthalmology at
"In terms of serious adverse events, one Durezol patient had chest pains unrelated to the drop," he says. "Other than that, two patients in each treatment arm had intraocular pressure increases." Five patients in the Pred Forte group (12.5 percent) were withdrawn due to lack of efficacy vs. none in the Durezol group, a difference that was statistically significant (p=0.01).
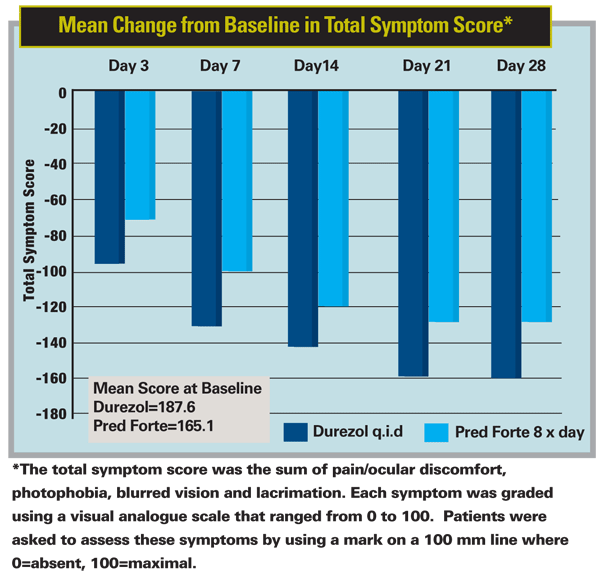
"What I tell my patients now is that this drug is comparable to Pred Forte [for uveitis]," says Dr. Flynn. "I'm not telling them it's better or worse. I'm a fellow who's prescribed Pred Forte for quite a while, though the thing I like is the four times a day vs. eight times. That's a benefit to my patients. More often, we're asking uveitis patients to go 12 or 14 times a day, so to be able to say eight to 10 times is a positive."
Barry Butler, president and chief executive officer of Sirion, says the company intends to submit this data to the U.S. Food and Drug Administration by the end of 2008. FDA approval of Durezol for the treatment of anterior uveitis could possibly be granted by the third quarter of 2009.
ISTA Files NDA for Bepreve
Ista Pharmaceuticals has filed a New Drug Application with the FDA for Bepreve (bepotastine ophthalmic solution), seeking its approval as an eyedrop treatment for ocular itching associated with allergic conjunctivitis. The company expects a standard review of 10 months from date of receipt.
In April, ISTA announced highly statistically significant reductions in the primary study endpoint of ocular itching from the preliminary analysis of its second and final Bepreve Phase III clinical study. In addition, the results showed Bepreve had a statistically significant effect on the rapidity of response and in certain secondary endpoints measuring additional signs or symptoms of ocular allergy, including improvement in nasal symptoms. There were no serious ocular adverse events reported in patients dosed with Bepreve from the study. Bepreve has three primary mechanisms of action: It is a non-sedating, highly selective antagonist of the histamine (H1) receptor; it has a stabilizing effect on mast cells; and it suppresses the migration into and activation of eosinophils in inflamed tissues. The compound's primary mechanisms of action are believed to make it an effective treatment against the signs and symptoms of allergic conjunctivitis. Bepotastine was approved in
Generic Glaucoma Drugs Approved
The FDA has begun approving generic equivalents of Merck's Cosopt and Trusopt. Hi-Tech Pharmacal Co. and Prasco were granted final approval of an Abbreviated New Drug Application for their dorzolamide and timolol ophthalmic solution. The product is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to beta blockers. The companies' ANDAs were also approved for dorzolamide ophthalmic solution 2%, the generic for Trusopt. Both plan to launch the generics immediately.

AMO: Healon Recall Limited to Single
Advanced Medical Optics recently received reports that a few
AMO's investigation has ascertained that this potential issue was restricted to a single lot of Healon D viscoelastic, which has been located and removed from inventory. All other Healon viscoelastic products are unaffected by this action. Healon D viscoelastic has been available in
Drug Shows Promise Against Non-healing Ulcers
RegeneRx Biopharmaceuticals announced that its ophthalmic drug candidate, RGN-259, was used to treat four neurotrophic keratitis patients with non-healing eye ulcers caused primarily by the herpes zoster virus under a Compassionate Use Investigational New Drug application. RGN-259 is a sterile eyedrop formulation of TB4 being developed by RegeneRx for use in treating ophthalmic wounds and related disorders.
Steven Dunn, MD, a corneal specialist and the sponsor/principal investigator of the



