For ophthalmologists, the eye is the center of the world, but they also need to remember to take a step back and look at how it connects to other tissues and organs, such as its link to the nose. Keeping their interconnected nature in mind can aid in making a diagnosis, treating a condition and preserving overall patient health. Here we will examine the connection between eye and nose, the specific anatomy linking the two, and some of the ways this connection can affect patients.
Traveling from Eye to Nose
We'll take a moment here to review the exact pathway tears take to travel from eye to nose. Once tears have been secreted from the main and accessory lacrimal glands and distributed evenly over the ocular surface by the eyelids, they are then passed into the nose through the lacrimal drainage system beginning with the upper and lower puncta. Each of these drains into a canaliculus, which carries the tears through the ampulla, the short vertical section of this tube, until turning towards the nose in a horizontal direction. The canaliculi may then join into a common canaliculus or enter the lacrimal sac separately.
Perhaps the most amazing mechanism of this path is the movement of tears into the nose via an active lacrimal pump assisted by the action of surrounding muscle groups (pretarsal and preseptal orbicularis oculi). When the eye is open, the normal state of the puncta and canaliculi is also open but the lacrimal sac is empty or collapsed. When a blink occurs, the surrounding muscles contract in such a way that the canaliculi are "pinched" closed. Simultaneously the collapsed walls of the lacrimal sac are drawn away from each other so that the sac is opened, creating a negative pressure and pulling the tears trapped in the canaliculi toward it. Then, as the eye reopens, the walls of the lacrimal sac return to their collapsed state, pushing the tears that have collected there downward to the nasolacrimal duct, eventually draining through the inferior turbinate to the nose.
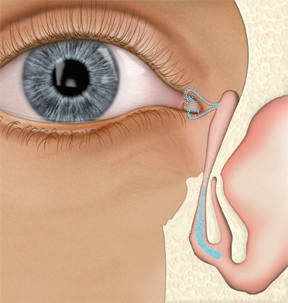 |
| When the eye is open, the normal state of the puncta and canaliculi is open, but the lacrimal sac remains collapsed until the next blink. |
These structures between eye and nose consist of connective tissue fibers in helical structural arrangements. Research has found that the lining of the nasolacrimal duct has a layered structure, similar to the tear film.1 Laboratory study into the expression of mucins in the lacrimal sac and nasolacrimal ducts has revealed that the efferent tear ducts produce a range of mucin types, and researchers have identified mRNA for a variety of mucins in human lacrimal sacs and nasolacrimal ducts. The authors hypothesize that this spectrum of mucins may aid in the flow of tears and defense against microbes.2
Medication Administration
Topical ocular administration of medication is often the best choice for treatment of localized disease symptoms. When instilling an eyedrop, the medication will reside briefly on the ocular surface, and then primarily drain through the pathway indicated above. Thus, it is easy to see how a portion of ophthalmic medication delivered to the surface of the eye could blend with tears and drain to the nose. It's highly unlikely, however, that a nasally administered rhinitis medication could reach the ocular surface via a direct route.
With nasally administered medications, typically in a spray form, there are more obstacles to delivery, including gravity, mucous and cilia in the nose and the convoluted nature of the nasal cavity.
Studies evaluating the distribution of nasal sprays often use radioactively labeled spray to quantify delivery to a certain region of the nose. One such study indicated that the delivery by spray varies widely, as measured by placing a small absorptive surgical pad at the middle meatus. The study found that 0.3 to 39.5 percent of the spray reached as far as the middle meatus/middle turbinate.3 The amount of drug reaching beyond this point would primarily be drained to the throat by nasocilliary clearance. It would be virtually impossible for a nasally administered drug to reach the eye directly, as the vast majority of a drug does not reach that high and what does is cleared downward to the throat. Even barring these two considerations, the unidirectional mechanism of the lacrimal pump (i.e., the flow of tears from the eye to the lacrimal sac and down to the inferior turbinate) prevents migration of any nasal medication upward via this pathway.
Although we know the direct movement of medication from nose to eye is highly improbable, topical nasal medication can be absorbed systemically via either local absorption through the highly vascular nasal mucosa into the circulation or via swallowing a portion of drug and subsequent absorption along the gastrointestinal tract.4 The systemic absorption can vary by drug, depending on properties such as lipophilicity. Similarly, some studies indicate that, as an eyedrop travels from eye to nose, a portion can be absorbed by the nasolacrimal ducts to potentially exert direct systemic effects.5 An example of this is the glaucoma agent timolol, which, when instilled in the eye, can induce bradycardia. Patients are often instructed to occlude the puncta when instilling timolol to minimize systemic absorption. This principle has been demonstrated in study using the Jones test, which administers fluorescein ocularly. The results of this study showed that when fluorescein is applied to the eye, it becomes apparent in the nose in 100 percent of patients within five minutes. However, when a modified Jones test was performed by instilling fluorescein in the nose via a nasal spray, no patients show fluorescein in the eye within five minutes.6
Along similar lines, when an ocular challenge with allergen is compared to a nasal allergen challenge, the spectrum of rhinoconjunctivitis signs and symptoms differs significantly. The ocular instillation of allergen to sensitized individuals' eyes yields clinically significant ocular and nasal symptoms. Nasal challenge with allergen yields only meaningful nasal symptoms.
A number of seasonal allergic rhinoconjunctivitis studies have observed the ability of ocularly administered anti-allergy medication to aid in the attenuation of nasal allergic signs and symptoms. For example, several such studies have been performed with the anti-allergy agent olopatadine (Patanol, Alcon): When an allergen challenge study paired olopatadine with fluticasone nasal spray (Flonase, GlaxoSmithKline) the attenuation of nasal symptoms was significantly greater than when the nasal spray was paired with a systemic antihistamine. And in an environmental study evaluating olopatadine alone, significant reductions in rhinorrhea, sneezing and postnasal drip were evident at several time points.7
These results are consistent with the known pathway of tear drainage, allowing a portion of the active medication to drain to the nose and confer some efficacy there. Another possibility for allergy is that ocularly administered medication essentially curbs the large outflow of mediators from degranulated mast cells, allowing fewer pro-allergy molecules to drain to the nose, thus indirectly controlling the nasal reaction to some degree. Either or both of these effects may be occurring.
Connections with Conditions
Due to the interconnected nature of the eye and nose, it is important to remember that, in some cases, the nose may provide the tip-off you need to recognize an ocular condition or a side effect of a medication, and vice versa for the eye. In addition, the border-region between the two—the nasolacrimal ducts—may also be the source of disease symptoms that manifest in the eye or nose.
Intraocular pressure elevation is an effect of systemic corticosteroid use that has also been documented with the use of nasal corticosteroids, and clinicians should watch for it closely in patients using these types of corticosteroids.8 This elevated IOP is most likely caused by systemic absorption of this locally administered medication. There are also several documented cases of effects on vision with steroids that are administered to the nasal region not by spray but by injection. Case reports have documented instances of both intraturbinate and subcutaneous nasal steroid injections resulting in vision loss.9,10 One set of case studies in a retrospective analysis revealed six patients who had been chronically administering corticosteroids in the form of inhalers or nasal sprays and developed central serious chorioretinopathy. Researchers hypothesized that the absorption of these corticosteroids was a likely factor in the development of CSC in these cases, particularly three cases that had a close temporal correlation between the use of the drug and development of CSC.
Several of the patients were also taking inhaled adrenergic agents, and the authors indicate that further study would be necessary to determine if these were a factor as well.11
One interesting study documents a case in which unusual nasal signs were the first indication of a problem with an ocular medication. Practitioners noted observation of brown or black papules located on the nasal mucosa and over the chin of a patient who had glaucoma and administered ophthalmic medications for this condition. Biopsy revealed sarcoidal granulomas and further analysis indicated these had a high sulfur content, however no signs of systemic sarcoidosis were present. Considering the location of the papules and the patient's glaucoma medications, two of which contained sodium bisulfate, the eyedrops were considered the most likely culprit as the causative agent of these pigmented papules.12
Epiphora, purulent secretions, and/or swelling of the nasolacrimal sac (which can sometimes appear similar to an orbital mass) may all be pointing to problems in nasolacrimal drainage for which the clinician should seek the underlying cause. Causes may include inflammation, trauma, tumor or a congenital defect in the drainage system.13,14
Dacryostenosis and dacryocystitis are perhaps the most common of these possible causes of problems in the nasolacrimal system, often presenting unilaterally. Epiphora is typically the hallmark symptom, logically, as tears cannot drain through obstructed, inflamed or infected passages.15 One study found that inflammation originating at the eye or the nose can induce swelling of the lacrimal system's mucous membranes. This can be followed by a restructuring of the helical connective fibers and can result in occlusion of this lacrimal pathway.16
Though rare, it's also important not to forget the possibility of a tumor. A handful of studies have documented such cases. One patient presenting with epiphora and purulent rhinorrhea was actually found to have an oncocytoma in the lacrimal sac that extended to the nasolacrimal duct.17
Another report documents the insidious nature of lacrimal sac tumors, as the initial symptoms simply point to dacryostenosis or dacryocystitis. This case documented a patient in whom a tumor was located by chance during dacryostenosis surgery. Be particularly wary of a tumor in patients with chronic dacryostenosis.18 There is a handful of similar cases in which initial symptoms, such as epiphora, point toward dacryostenosis but eventually result in chance discovery of cancerous tissue during surgery.19
It's important to maintain an awareness of the close connection between the eye and nose: To some degree, what you are doing to the eye you are also doing to the nose, and what you're doing to the nose you're doing systemically.
The passages between eye and nose containing the canaliculi, lacrimal sac and nasolacrimal ducts represent something of a no man's land between the ophthalmic and the ear, nose and throat specializations, but maintaining vigilance on both ends can ensure the appropriate identification and treatment of problems in the middle.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Dr. Metson is a clinical professor at Harvard Medical School, and a member of the department of otolaryngology at Massachusetts Eye and Ear Infirmary. Ms. Fink is manager of medical communications at Ophthalmic Research Associates.
1. Paulsen F. The human nasolacrimal ducts. Adv Anat Embryol Cell Biol 2003;170:III-XI, 1-106.
2. Paulsen F, Corfield AP, Hinz M, et al. Characterization of mucins in human lacrimal sac and nasolacrimal duct. Invest Ophthalmol Vis Sci. 2003;44:5:1807-13.
3. Homer JJ, Maughan J, Burniston M. A quantitative analysis of the intranasal delivery of topical nasal drugs to the middle meatus: spray versus drop administration. J Laryngol Oto. 2002;116:1: 10-13.
4. Szefler SJ. Pharmacokinetics of intranasal corticosteroids. J Allergy Clin Immunol 2001;108:1: S26-S31.
5. Paulsen FP, Foge M, Thale AB, Tillman BN, Mentlein R. Animal model for the absorption of lipophilic substances from tear fluid by the epithelium of the nasolacrimal ducts. IOVS 2002;4310: 3137-3143.
6. Spangler DL, Abelson MB, Ober A, Gomes PJ. Randomized, double-masked comparison of olopatadine ophthalmic solution, mometasone furoate monohydrate nasal spray, and fexofenadine hydrochloride tablets using the conjunctival and nasal allergen challenge models. Clin Ther 2003; 25:8:2245-2267.
7. Abelson MB, Turner D. A randomized, double-blind, parallel-group comparison of olopatadine 0.1% ophthalmic solution versus placebo for controlling the signs and symptoms of seasonal allergic conjunctivitis and rhinoconjunctivitis. Clin Ther 2003;25:3:931-946.
8. Opatowsky I, Feldman RM, Gross R, Feldman ST. Intraocular pressure elevation associated with inhalation and nasal corticosteroids. Ophthalmology 1995;102:2:177-9.
9. Martin PA, Church CA, Petti GH Jr, Hedayi R. Visual loss after intraturbinate steroid injection. Otolaryngol Head Neck Surg 2003;128:2:280-1.
10. Shafir R, Cohen M, Gur E. Blindness as a complication of subcutaneous nasal steroid injection. Plast Reconstr Surg 1999;104:4:1180-2.
11. Haimovici R, Gragoudas ES, Duker JS, Sjaarda RN, Eliott D. Central serious chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology 1997;104:10:1653-60.
12. Carlson JA, Schutzer P, Pattison T, Del Rosario A, Mihm MC Jr. Sarcoidal foreign-body granulomatous dermatitis associated with ophthalmic drops. Am J Dermatopathol 1998;20:2:175-8.
13. Deitrich C, Mewes T, Kuhnemund M, Hashemi B, Mann WJ, Amedee RG. Long-term follow-up of patients with microscopic endonasal dacryocystorhinostomy. Am J Rhinol 2003;17:1:57-61.
14. Yen MT, Hipps WM. Nasolacrimal sac hematoma masquerading as an orbital mass. Ophthal Plast Reconstr Surg 2004;20:2:170-2.
15. McEwan DR. Surgical treatment of dacryocystitis. AORN J. 1997 Aug; 66(2): 268-70: 273-8.
16. Paulsen FP, Thale AB, Maune S, Tillman BN. New insights into the pathophysiology of primary acquired dacryostenosis. Ophthalmology 2001; 108:12:2329-36.
17. De Bree R, Scheeren RA, Kummer A, Tiwari RM. Nasolacrimal duct obstruction caused by an oncocytoma. Rhinology 2002;40:3:165-7.
18. Hung SL, Ma L. Recurrent lacrimal sac papilloma: Case report. Changgeng Yi Xue Zhi. 2000;23: 2:113-7.
19. Zehavi C, Zadok J, Sachs D. The pitfall of dacryostenosis. Ophthalmic Surg 1992;23:4:297-8.



