Thyroid eye disease can be an extremely distressing condition that affects both men and women, primarily in their formative years. Fortunately, our understanding of the disease process has increased dramatically of late, allowing for targeted treatments that have the potential to change the course of the disease. Here, I’ll discuss the epidemiology of TED, the anatomy involved, how it affects the eye and how to best diagnose it.
Pathophysiology
The thyroid gland, located in the neck, is chiefly responsible for secreting thyroid hormones. These hormones are integrally involved in almost every process of the body, including regulation of the heart, lungs, brain and metabolism.
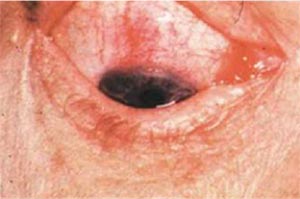 |
| Figure 1. Superior limbic keratopathy in early inflammatory thyroid disease, highlighted by rose bengal stain. |
If the thyroid produces too much of the hormone thyroxine, hyperthyroidism occurs. Hyperthyroidism leads to cell growth, both via the effects of the hormone and via the IGF-1 (insulinlike growth factor) pathway, which is connected to the thyroid hormone receptor. Activation of these two pathways leads to growth of fat, muscle and fibrous cells, causing the characteristic clinical manifestations of thyroid eye disease. There are many obvious effects of hyperthyroidism, such as increased heart rate with palpitations, increased respiratory rate, diarrhea and increased metabolism leading to resorption of bone and fat. Hypothyroidism can also occur, leading to fatigue, weight gain or gastric dysfunction. In addition, equally important but subtler symptoms can occur in both kinds of thyroid disorder, such as cognitive dysfunction, depression, poor memory, numbness and tingling.
Thyroid disorders are generally diagnosed by detecting abnormalities in a patient’s blood work. Thyroid-stimulating hormone (TSH) and thyroid hormones (T3, T4) can be altered in ways that allow the physician to tell if the body is secreting more or less hormone than normal. During treatment, these hormone levels may also be tracked to determine if the therapy is controlling the hormone levels.
In general, between 25 and 50 percent of patients with a thyroid problem (hyperor hypothyroidism) will go on to develop some form of TED,1 with roughly 5 percent having a moderate to severe form of the disease that can affect vision. Interestingly, once TED starts, it’s a separate process from the initial thyroid abnormality, with very little interaction between the two. Specifically, once patients develop TED, having wellcontrolled thyroid hormone levels won’t make the eye disease better, although having poorly-controlled thyroid hormone levels may make it worse. Though strides are constantly being made, it’s still unclear exactly why patients with a thyroid-hormone abnormality develop the eye disease.
Epidemiology
Different factors influence a patient’s development of TED:
• Type of thyroid disorder. While the majority of patients who develop TED are hyperthyroid (~80 percent), a small percentage are hypothyroid (~10 percent), and an even smaller percentage (5 to 10 percent) may be euthyroid, without any previously diagnosed thyroid abnormality. The disease is primarily a young person’s disease, affecting patients in their 30s, although there is another peak in the late 60s.2 It disproportionally affects young women, for whom it can be mild, though if young men or older men and women develop the disease they tend to have a more severe course.2,3
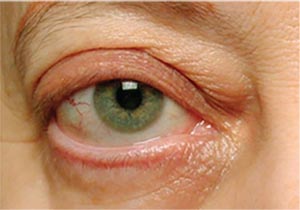 |
| Figure 2. Exposure keratopathy from lower eyelid retraction in TED’s stable phase. |
TED results in changes to the eyes’ appearance, and since the eyes and face are the most unique and scrutinized part of the body, any changes to them—particularly in young adulthood when patients are advancing in their careers and starting families—can be particularly distressing. Fortunately, the disease spontaneously improves or stabilizes in the great majority of patients, with only a very small percentage (~15 percent) experiencing progressive worsening.4
Dr. Francis Rundle first described the clinical course of TED in the early 20th century, with a famous graph depicting two phases of the disease: active and stable. Dr. Rundle observed that TED tends to be “active” at first, with inflammation and worsening for the first 18 to 36 months of the disease, followed by a gradual improvement into a “stable” phase.1,5 Roughly 10 percent of patients may have reactivation of disease at some point in their life, and this may be precipitated by surgery. Older patients may not have a pronounced active phase with severe inflammation, but may have a slow, subtle and fibrotic course that’s very difficult to treat.
In general, treatment centers around managing inflammatory symptoms and improving quality of life in the active phase, and surgery to restore the cosmetic and functional outcomes in the stable phase.
• Genetics. Genetic factors clearly play a role in the development of TED. Graves’ disease itself is known to have a hereditary component; twin studies have shown that monozygotic (identical) twins have a much higher incidence of Graves’ (~30 percent) compared to dizygotic (fraternal) twins, at 3 percent.6 Furthermore, when asymptomatic family members of patients with TED were examined, many had early signs of TED without any systemic thyroid abnormalities.7 Scientists are spending a great deal of time identifying which genes in particular may cause someone to be susceptible to developing TED, in the hopes that this may help with prognosis and early treatment. However, the specific genetics of TED are still poorly understood.
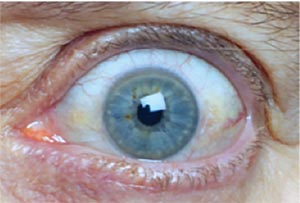 |
| Figure 3. Upper eyelid retraction causing abnormal opening of the eyes. |
• Environmental factors. Smoking is perhaps the biggest environmental risk factor that can trigger TED. TED is a disease of orbital inflammation, and the additional inflammatory molecules in tobacco smoke have been proven to significantly worsen disease8 by up to 700 percent, possibly due to the creation of free radicals and reactive oxygen species. This risk is directly related to the number of cigarettes smoked and is reversible—smoking cessation can lead to milder disease and visual improvement—so it is never too late to stop.
The gut microbiome has also been implicated in TED pathogenesis. Certain bacteria that colonize the intestines, such as Yersinia enterocolitica, create proteins that appear to be very similar to thyroid-related proteins. When the immune system mounts an attack against such bacteria, the antibodies can cross-react with the thyroid gland and related tissues, leading to worsening of disease.9
• Nutrition factors. Finally, various vitamins and minerals may be deficient in patients who develop TED. Selenium, a trace mineral important in the function of anti-inflammatory proteins, has been found to be deficient in patients with Graves’ disease, hypothyroidism and TED,10 and selenium supplementation has been shown to reduce the symptoms of mild TED.11
Vitamin D is also reduced in many autoimmune states, including Graves’ disease, and may also be related to an anti-inflammatory effect that’s still unclear.12 Either a pre-existing vitamin deficiency has caused the development of TED, or the development of TED has consumed these vitamins and minerals such that the low levels reduce the production of anti-inflammatory proteins. In either scenario, supplementation of these factors may improve the course of TED.
Other Factors
Circulating antibodies to various thyroid-related proteins are responsible for the manifestations of Graves’ disease, which results in hyperthyroidism. There have been several welldescribed antibodies that are relevant both to systemic Graves’ and TED, including anti-TSHR (thyroid-stimulating hormone receptor), anti-TPO (thyroid peroxidase) and anti-Tg (thyroglobulin) antibodies. These antibodies target thyroid-related proteins which are present both in the thyroid gland and the orbit, as well as other connective tissues in the body such as the shins (leading to swelling, or pretibial myxedema).
Antibodies against TSHR are particularly important and can be further categorized as TSHR-binding (TBII), TSHR-stimulating (TSI) or TSHRblocking.13 TSI seem to be particularly important, as the levels of TSI are directly correlated with clinical severity in TED, with a level greater than 400 suggesting moderate-to-severe disease.14,15 TSI may also help predict the development and severity of TED in both adults and pediatric patients.16,17
Hyperthyroid Treatment
Physicians have several tools to treat hyperthyroidism, including oral antithyroid medicine (methimazole, propylthiouracil), radioactive iodine and surgical thyroidectomy. Oral medicine blocks formation of hormones in the thyroid, but doesn’t alter antibody levels. Given the importance of TSI in the development of TED, it stands to reason that oral anti-thyroid medicine doesn’t prevent the development of TED. Radioactive iodine treatment consists of administration of an iodine molecule that’s absorbed by any cell that imports iodine internally, i.e., cells in the thyroid gland and the orbit. This leads to a powerful inflammatory response that can both worsen existing TED and increase the probability of development of TED in patients who don’t have any ocular manifestations.18 Pre-treatment with steroids can reduce the risk of worsening TED.19
Surgical thyroidectomy is removal of the entire thyroid gland by a qualified head-and-neck surgeon. Risks such as vocal cord dysfunction, persistent calcium abnormalities or intraoperative bleeding or thyroid storm are low, and thyroidectomy may provide the quickest route to control of the thyroid hormones. While having excellent thyroid hormone control doesn’t improve the course of TED, having poor thyroid hormone control may worsen TED (possibly by increasing the TSI level), so quickly and consistently achieving control of thyroid hormone levels is important.
Clinical Manifestations of TED
Thyroid eye disease has several characteristic manifestations, although the disease is notoriously asymmetric and variable in presentation. By far, ocular surface disease and eyelid changes are the most common, present in more than 90 percent of patients with TED. Proptosis is present in 70 to 80 percent of patients, and strabismus (extraocular muscle changes with double vision) occurs in roughly 50 percent of patients. True sight-threatening disease is rare, on the order of 2 to 5 percent. Following is a detailed discussion of these manifestations.
• Ocular surface disease. Ocular surface disease encompasses all symptoms that relate to poor function of the eyelid-tear-cornea interface. These symptoms can include light sensitivity, gritty or painful eyes, tearing and blurry vision when reading, driving, working on the computer or watching TV. These symptoms may be worsened in windy, dry or low-humidity conditions.
Ocular surface disease in TED has two main causes: ocular inflammation and ocular exposure. In the first 12 to 18 months of TED, when the disease is in the active phase, inflammatory molecules can attack the mucous-producing cells, tear gland and the corneal surface, and cause breakdown of the tear film (Figure 1). In the stable phase, inability to close the eye completely due to eyelid changes can lead to chronic exposure of the inferior portion of the cornea, leading to dryness (Figure 2). It’s important to distinguish between the two causes, as the treatments differ. Inflammatory dry eye demands control of the inflammation, either via control of the thyroid disease itself or with topical steroid drops. Dry eye from chronic exposure can be treated with artificial tears and other forms of ocular lubrication, as well as orbital decompression or eyelid surgeries. The two types of dry eye often coexist, and it’s important to perform a careful evaluation to address each patient’s unique condition.
• Eyelid. Thyroid eye disease primarily causes upper and/or lower lid retraction
(Figure 3). This disturbs the normal blink reflex and can lead to inability to close the eye, or lagophthalmos (Figure 4). This is caused by fibrosis, or scarring in the muscles that maintain the eyelid position. Some form of eyelid retraction is present in more than 90 percent of patients with TED, and it can be very asymmetric. Eyelid surgery can often improve the cosmetic and functional aspects of these eyelid changes.
• Extraocular muscles. Strabismus, or misalignment of the eyes, is a common finding in TED, and is present in roughly half of patients (Figure 5). Enlargement of the extraocular muscles that move the eyeball leads to inability of these muscles to relax, pulling the eye away from the neutral gaze. Double vision resulting from strabismus can be one of the most debilitating consequences of TED, impairing the ability to drive, read or work. Pressure on the eyeballs from these enlarged muscles can also lead to increased intraocular pressure and glaucoma. Strabismus surgery or prisms in spectacles can help improve double vision, although a small amount of diplopia in extreme positions of gaze often remains.
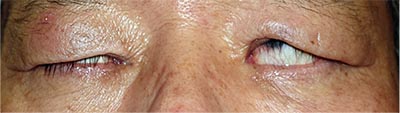 |
| Figure 4. A possible symptom of thyroid eye disease is the inability to close the eyes even with forceful blinking, known as lagophthalmos. |
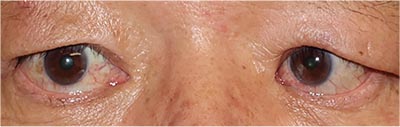 |
| Figure 5. Inwardly crossing left eye (esotropia) as a result of thyroid eye disease. |
• Orbit. Growth of fat, muscle and fibrous tissue leads to an increased volume of orbital soft tissue, while the bony confines of the orbit don’t change. Like a scoop of ice cream on a cone that’s too small, the eyeball can be pushed forward, known as proptosis (Figure 6). This bulging of the eyes is cosmetically dis figuring and causes dry eye, eye pain and other disturbances in visual function. The overall tightness of the orbital tissues also causes congestion and poor blood flow, which leads to swelling, redness and a dull ache behind the eye (Figure 7). If the tightness is severe enough, patients may even experience vision loss due to corneal ulceration or lack of blood flow to the optic nerve. Patients need a combination of medical and surgical therapies for rehabilitation after these conditions develop.
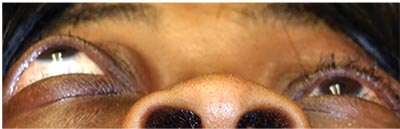 |
| Figure 6. Proptosis, or bulging of the right eye, due to growth of tissue behind the eyeball in thyroid eye disease. |
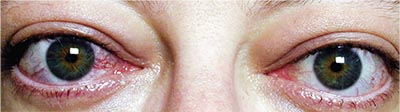 |
| Figure 7. Orbital congestion with redness, swelling and retrobulbar ache in thyroid eye disease. |
Currently, a thorough understanding of the molecular basis of thyroid eye disease has allowed for earlier diagnosis with more accurate prognostication. Much research still needs to be done, however, since it’s unclear exactly which factors trigger the onset of TED in a patient with Graves’ hyperthyroidism. Elucidating these factors may allow accurate prediction and allow for earlier treatment. In Part 2 of this series on thyroid eye disease, which will appear in the next installment of Plastic Pointers, we’ll delve into ways of managing TED. REVIEW
Dr. Ramesh is a clinical assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University, and a member of the Wills Eye Hospital Orbital and Oculoplastic Surgery Service. He practices at Eye and Facial Plastic Surgery Consultants in Langhorne, Pennsylvania. His areas of interest include TED, facial trauma and cosmetic facial and eyelid procedures.
1. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci 2014;55:3:1735-48.
2. Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc 1994;92:477-588.
3. Bartley GB, Fatourechi V, Kadrmas EF, et al. Chronology of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 1996;121:4:426-34.
4. Shan SJ, Douglas RS. The pathophysiology of thyroid eye disease. J Neuroophthalmol 2014;34:2:177-85.
5. Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci 1945;5:3-4:177-94.
6. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: A population-based study of two Danish twin cohorts. J Clin Endocrinol Metab 2001;86:2:930-4.
7. Ardley M MT, Lahooti H, Champion B, Wall JR. Eye findings and immunological markers in probands and their euthyroid relatives from a single family with multiple cases of thyroid autoimmunity. Thyroid Res 2012;5:4.
8. Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med 1993;329:20:1468-75.
9. Luo G SG, Niesel DW. Purification and characterization of Yersinia enterocolitica envelope proteins which induce antibodies that react with human thyrotropin receptor. J Immunol 1994;152:2555-61.
10. Liu Y, Liu S, Mao J, et al. Serum trace elements profile in Graves’ disease patients with or without orbitopathy in northeast China. Biomed Res Int 2018;2018:3029379.
11. Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of mild Graves’ orbitopathy. N Engl J Med 2011;364:20:1920-31.
12. Planck T, Shahida B, Malm J, Manjer J. Vitamin D in Graves disease: Levels, correlation with laboratory and clinical parameters, and genetics. Eur Thyroid J 2018;7:1:27-33.
13. Diana T, Kahaly GJ. Thyroid stimulating hormone receptor antibodies in thyroid eye disease-methodology and clinical applications. Ophthalmic Plast Reconstr Surg 2018;34:4S (Suppl 1):S13-S9.
14. Lytton SD PK, Kanitz M, et al. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab 2010;95:2123-31.
15. Ponto KA, Kanitz M, Olivo PD, et al. Clinical relevance of thyroid-stimulating immunoglobulins in Graves’ ophthalmopathy. Ophthalmology 2011;118:11:2279-85.
16. Kampmann E DT, Kanitz M, et al. Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid-associated orbitopathy: A prospective study. Int J Endocrinol 2015;2015:678194.
17. Diana T BR, Bossowski A, et al. Clinical relevance of thyroid-stimulating autoantibodies in pediatric Graves’ disease a multicenter study. J Clin Endocrinol Metab 2014;99:1648-55.
18. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med 1998;338:2:73-8.
19. Tallstedt L, Lundell G, Torring O, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. The Thyroid Study Group. N Engl J Med 1992;326:26:1733-8.



