Posterior capsule opacification can threaten vision postoperatively, but this can be avoided simply with a YAG capsulotomy. What’s challenging, however, is that PCO can develop over a long period of time and the severity levels may not increase for years. So, when’s the best time to YAG for PCO? Here, surgeons weigh in.
When to YAG
There are many arguments for why surgeons should perform an Nd:YAG laser capsulotomy early after cataract surgery because the risk of PCO fluctuates over time.
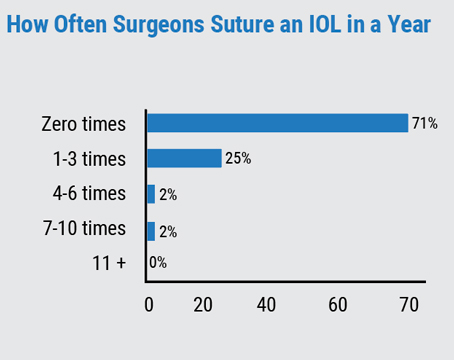
“There are almost four million cataract surgeries done in the U.S. every year, and the estimates are that roughly 10 to 30 percent of cataract surgery patients will develop PCO,” shares J. Kevin McKinney, MD, an ophthalmologist practicing at Eye Health Northwest in Oregon City, Oregon. “It depends on the type of implant and the surgical technique used, but it does become more common with time. So, it’s something we see almost every day.” One large population study1 showed that the incidence of PCO after three years following surgery ranged between 4.7 and 18.6 percent, which increased to between 7.1 and 22.6 percent after five years. Also, the incidence of Nd:YAG capsulotomy increased at three and five years. So we know how prevalent PCO is following cataract surgery, and the rate at which it develops. Knowing this, should physicians employ YAG earlier?
“Technically you could do a posterior capsulotomy very quickly after surgery as long as the capsule has become tight,” explains Dr. McKinney. “When that happens, it becomes tight and it’s easier to cut. So as soon as it’s become tight, you could do the laser.” This occurrence has been studied before and physicians are aware that it can take some time for patients’ capsules to reach complete contact with their lens implants. It was discovered that silicone IOLs following implantation take approximately eight days to gain full contact with the capsule and 11 days for acrylic IOLs to hit the same mark.2 By these metrics, ophthalmologists could perform an Nd:YAG capsulotomy within the first two weeks post-cataract surgery. But some physicians argue that this may be too soon to employ an Nd:YAG capsulotomy.
“I like to wait to do a YAG laser capsulotomy after cataract surgery until the blood-aqueous barrier has reestablished, meaning until any chance of postoperative inflammation following that cataract surgery goes away,” says Nick Mamalis, MD, the director of the Ophthalmic Pathology Laboratory and a professor of ophthalmology & visual sciences at the University of Utah School of Medicine. “And so, depending on the patient and depending on what you read in the literature for reestablishment of the blood-aqueous barrier after cataract surgery could be on the order of six, eight, even 12 weeks following surgery. So, I’m not one to do a really early YAG laser capsulotomy. I want to make sure everything is completely healed and reestablished before jumping in and doing a capsulotomy.”
 |
|
Posterior capsule opacification at different severity levels based on a research study.7 Grade 0 indicates no opacity, or opacification was found only on the peripheral capsule. Grade 1 indicates that the posterior polar retina can be clearly viewed, but some capsular wrinkling or opacity was found centrally and peripherally. Grade 2 indicates opacification worse than Grade 1, but the cup-to-disc ratio isn’t affected and can still be observed. Grade 3 indicates the first stage of PCO with a visual impact. Patients with Grade 3 PCO have worse opacification than Grade 2, and the cup-to-disc ratio is difficult to observe. Grade 4 is the most severe case of PCO. Although Grade 4 opacification is similar to Grade 3, fundus observation is far more difficult, and in some cases, impossible. (Creative Commons License: https://creativecommons.org/licenses/by-nc/4.0/.) Photo: Gu X et al. |
As Dr. Mamalis suggested, depending on the literature, the rate at which the blood-aqueous barrier can fully heal differs. Earlier studies3,4 found that the blood-aqueous barrier reached normalcy at approximately three months following surgery, and those investigators employed extracapsular cataract extraction. However, one study5 compared phacoemulsification to ECCE and discovered that a significant number of their cohort that underwent phaco regained a healthy blood-aqueous barrier within two to five weeks, while it took four to six weeks for ECCE patients to meet the study’s endpoint.
“Just like with cataract surgery, the main determinant of when it’s time to do YAG capsulotomy is the patient’s visual function,” adds Dr. McKinney. “So not just that their vision is reduced, but that they have a functional complaint—difficulty reading or driving at night, for example. When that develops, as long as enough time has passed after surgery, it’s a reasonable time to do the laser. It’s true, just like with a regular cataract, if you let it get super thick, then it’s harder to open and complications can be more likely. So, there’s an essence of timeliness, but it’s not like you have to jump on it as soon as it develops. You really want to wait until a patient has a functional visual deficit.”
One point that Dr. McKinney made was that PCO could become “super thick,” or severe. Earlier in this article, a study was presented looking at the incidence rates of PCO over a three- and five-year period, but this study failed to observe early-onset PCO and severity levels in their study’s population. Early-onset PCO can develop in about three months following cataract surgery, and if it’s not treated properly, then it can cause light scattering and reduce visual performance over time. Approximately 3 percent of patients may experience severe levels of PCO during early development of the condition.6 The severity levels can be graded from 0 to 4, where grades 3 and 4 are defined as having PCO with a visual impact.7 This can alter the timeframe in which an Nd:YAG capsulotomy is performed, since the severity of PCO may not have as much of an impact on a patient’s visual function early on. But don’t wait too long.
“If you have a really significant PCO, especially what we call a fibrotic PCO where the lens capsule has become very stiff and very fibrotic, or if you have PCO caused by a large amount of proliferation of cortical material, then the denser the material and the denser the PCO, and the more difficult it is to perform the YAG laser capsulotomy,” shares Dr. Mamalis. “And so, you always want to try to do the capsulotomy as soon as necessary without waiting an inordinate amount of time until the capsulotomy itself becomes more difficult to do.”
 |
|
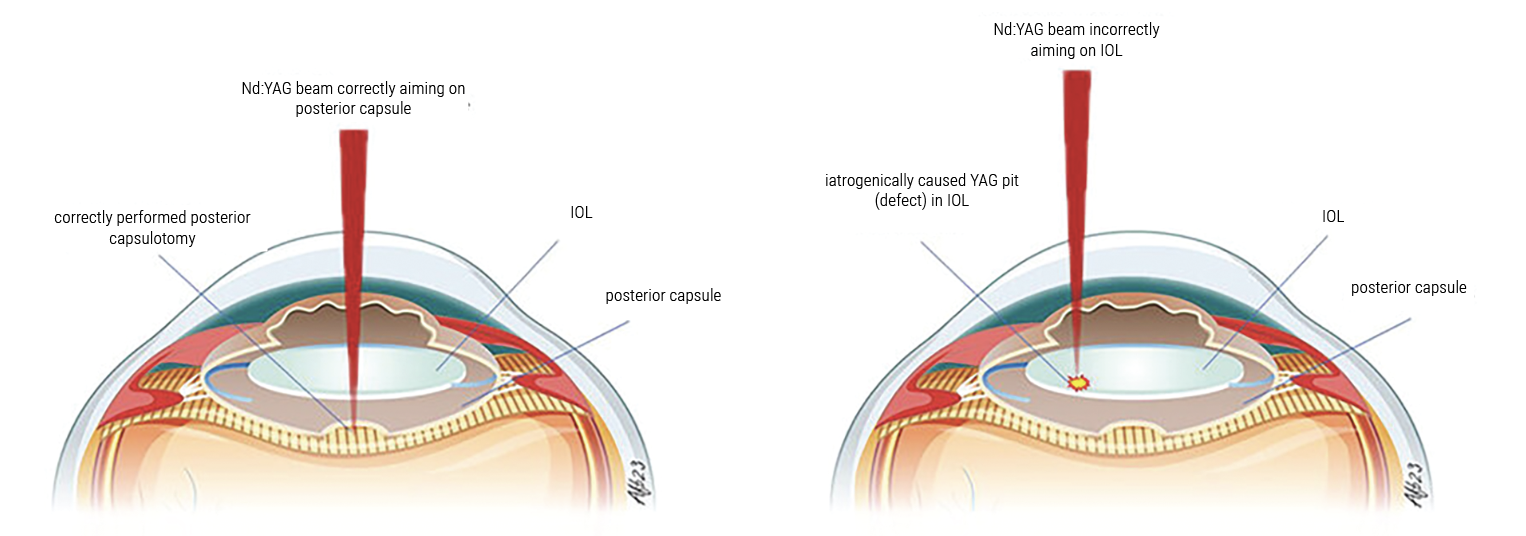
Graphic depicting an Nd:YAG laser beam being applied to an eye with an IOL implanted. Precise focus of the laser allows for safe and effective treatment (left), while incorrectly positioning the laser can pit the IOL (right). (Creative Commons License: https://creativecommons.org/licenses/by-nc/3.0/.) Photo: Andreas Borkenstein, MD. |
Complications of an Early YAG
“If you do the laser too soon, there are studies to show that there’s an increased risk of retinal tear or retinal detachment,” says Dr. McKinney. “There are certain risk factors that make that more common, such as in a young patient or someone who hasn’t had a posterior vitreous detachment yet. So yes, you can do it [early], but it’s generally accepted practice that you should attempt to wait a few months to perform the laser to reduce those complications.”
There are studies that look at the rate at which retinal tears and detachments occur following an Nd:YAG capsulotomy, but none has fully assessed the risk of these conditions developing. Of course, there’s literature that could bolster the argument that the procedure increases the risk of developing vitreoretinal diseases, but these studies have varying population sizes and patient demographics as well as subjects with comorbidities that could contribute to retinal tears and detachment. One study reviewed literature on the subject but couldn’t find conclusive evidence that the laser increases these risk factors, but they do believe that the laser’s energy should be as low as possible to reduce the chance of anterior hyaloid damage, which may increase the chance of developing vitreoretinal diseases.8
Dr. Mamalis says he uses the re-establishment of the blood-aqueous barrier as a gauge for the timing of an Nd:YAG capsulotomy, because he notes that complications could arise for the patient if treated prematurely. “There may be a rebound of inflammation, or the laser and vaporizing of the capsule along with liberating the material that’s in the capsule could at least theoretically trigger more inflammation or a rebound of inflammation after the cataract surgery,” he explains. “There are some who advocate doing YAG laser right away, and as soon as the cornea is clear and the wound is stable, it’s okay to do it after cataract surgery. My own personal reasoning is that I like to wait for the blood-aqueous barrier to reestablish.”
There’s the possibility that an Nd:YAG capsulotomy could be detriment to the ocular surface and patient’s vision if done too early. Two studies from investigators in Taiwan examined refractive9 and corneal endothelial10 changes of two groups of patients—one group underwent a capsulotomy early on, within one year; one group underwent a capsulotomy later on, after one year.
Results from the refractive study9 showed that patients who underwent Nd:YAG capsulotomy within one year post-cataract surgery showed a similar significant improvement to CDVA compared to the later surgical group. However, earlier surgical patients revealed more significant hyperopic changes compared to the later surgical group, which correlated with a deeper axial chamber depth. Therefore, the investigators believe surgeons should wait more than a year before performing a capsulotomy.
Similarly, the results from the corneal endothelial study proved that waiting longer to perform an Nd:YAG capsulotomy can mitigate the reduction of endothelial cell density following surgery.10 Although their cell density levels recovered over time, the earlier surgical group showed a significant reduction after one week postop. However, their recovered status was still lower than the postoperative baseline results. Endothelial cell density in the earlier surgical group was recorded at 2,441.55 ±321.80 mm2 postoperatively, 2,221.50 ±327.73 mm2 at one week, and 2,369.95 ±341.53 mm2 at four weeks after laser capsulotomy. The later surgical group maintained their baseline parameters without a significant change throughout the four-week follow-up period (2,584.57 ±274.51 mm2 postoperatively, 2,565.14 ±304.66 mm2 at one week, and 2,557.32 ±317.87 mm2 at four weeks).
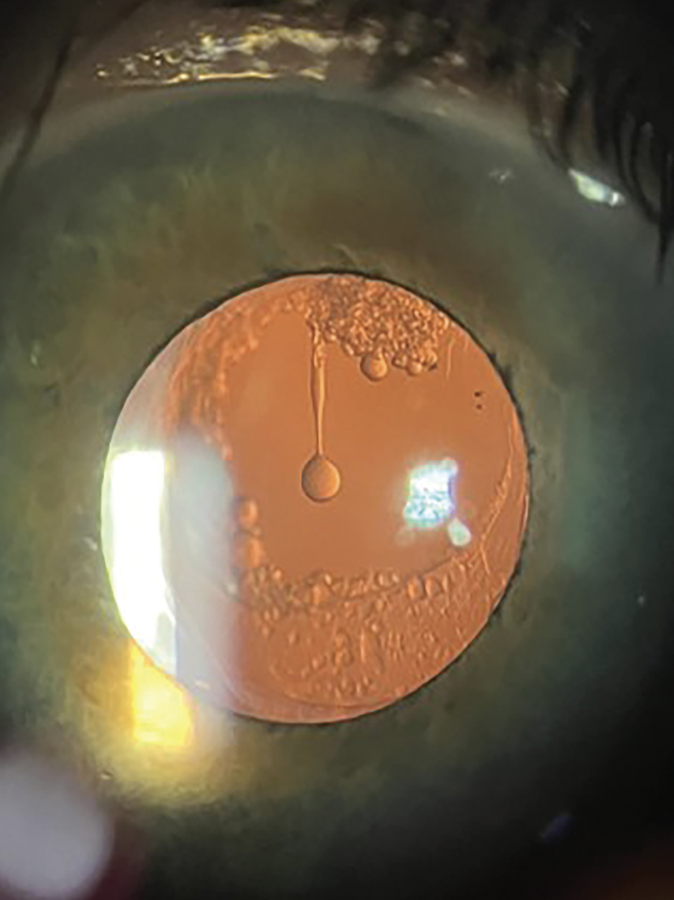 |
| Elschnig pearls can be found hanging in the posterior capsule. If an Nd:YAG laser is unavailable, peeling and aspiration of the pearls can be a safe and effective method to employ. (Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.) Photo: C.Y. Levis, MD; Kaleigh Haus. |
Surgical Pearls
Physicians beginning their first steps towards treating PCO should be aware of all possible outcomes that may arise during surgery. Many experienced surgeons tout that using an Nd:YAG laser to perform a capsulotomy is quick and easy, but there’s a learning curve young ophthalmologists should be aware of before handling the laser.
“Firing the laser is easy … you just need to push a button,” boasts Dr. McKinney. “But the challenge is that the YAG laser throws off shockwaves that can damage the eye and can pit or even crack an intraocular lens. So, you want that energy to cut the cloudy capsule to clear the visual axis, but you want to avoid collateral damage to the eye. It’s critical to be able to aim it very precisely. On average, the distance between the capsule and the implant lens is only 130 µm. That’s the length of a particle of dust. Sometimes the capsule lies right on the implant, so that increases the risk that you can damage the IOL when you do the laser.
“You also need to know where to put the laser spots,” he continues. “Depending on the nature of the cloudiness, there may be a pattern of laser spots that would be most effective at opening the capsule. In addition, you want to know how big to make the capsulotomy. If you make it too small, you may not remedy the patient’s visual complaints. If you make it too big, you may let vitreous come forward into the anterior chamber, or you may actually cause the implant to be unstable. It’s rare to have complications with a YAG laser if you’re an experienced surgeon, but those complications can be sight threatening. So, it’s essential to be able to recognize and manage complications, and that requires extensive training and experience.”
In addition to Dr. McKinney’s advice on how to properly perform an Nd:YAG capsulotomy, Dr. Mamalis mentioned that ocular abrasions and irregularities can reduce the effectiveness of the laser, as it won’t allow the ophthalmologist to properly focus the laser spots.
“If there’s any significant scarring in the cornea, then you’re not getting a clear view,” Dr. Mamalis adds. “That can make the YAG laser more difficult. If the patient has severe dry eyes where there are irregularities on the surface of the eye, that can also make it more difficult to do the laser. If you’re going to be doing YAG capsulotomy, you want to dilate the pupil as widely as you can, because doing a YAG capsulotomy through a smaller pupil or a pupil that is scarred down with synechiae makes it more difficult to do.”
Also, be aware of when not to employ an Nd:YAG capsulotomy. Yes, this procedure is the current mainstay for treating PCO, but sometimes patients’ complaints may have arisen from some other ocular complication.
“There are patients who’ve received multifocal IOLs who have significant dysphotopsias or other issues with their vision, and there are those in our profession out there who tout a very early immediate YAG laser capsulotomy to clear the visual access and get rid of these potential issues,” shares Dr. Mamalis. “I take the opposite tack. I say you definitely shouldn’t do a YAG laser capsulotomy in a patient who’s got visual aberrations and dysphotopsias from a multifocal lens because the reason for that is actually from the lens itself, not the posterior capsule.
 |
“Once you’ve opened a posterior capsule, if you have to do a subsequent IOL exchange, that exchange becomes much more difficult to do,” he continues. “And so, if you’ve got a patient who’s having issues postoperatively, but they’ve had cataract surgery, their implant looks fine, yet they’re having issues with glare, photophobia, dysphotopsia, other things like that, then you better make sure that that is coming from the capsule, not the lens itself before you do a YAG. So that’s the one time I would advocate taking a step back and saying, ‘Hey, wait a minute, should we really be doing a YAG capsulotomy and trying to improve this patient’s symptoms, or should we be looking at the lens itself as the culprit rather than the posterior capsule?’ ”
Although uncommon, Dr. McKinney noted that epithelial cells can regrow as either fibrous membrane on the back of the IOL or as Elschnig pearls. He explained that this is caused by the capsule being opened during the procedure. Membranous PCO doesn’t always have to be treated with an Nd:YAG laser, and peeling and aspiration of Elschnig pearls has been cited as an effective alternative to capsulotomy.11 However, this requires taking the patient into the operating room “A time when you might not want to use YAG would be if the zonules are very weak or missing,” adds Dr. McKinney. “For example, in a post trauma patient, sometimes you might actually dislocate the lens by doing the laser. In that setting, it may be better for a vitreoretinal colleague to do a pars plana vitrectomy and remove the back of the capsule. If the lens becomes unstable in that setting, they’re already in the eye and they can take the lens out and sew in a secondary implant lens.” (See Secondary IOL: Best Practices)
Additionally, physicians can attempt to prevent PCO by implanting specific IOLs following cataract surgery. Make sure to talk with the patient before making this choice for them, because they’re looking for comfort and improvement to their vision and may not want the IOLs that could potentially mitigate the development of PCO in terms of how they’re implanted.
“In terms of trying to prevent PCO, there are some different things that we can do with an IOL,” says Dr. Mamalis. “One of the things that’s been studied the most is to put what we call a sharp edge on the posterior optic of the IOL. And so, if you can put a sharp edge on there—it’s a right-angle band—then you can sometimes get the lens capsule to almost shrink wrap around the posterior surface of the IOL postoperatively.
“Anything you can do that creates a barrier where the capsule meets the edge of the lens optic can help to slow down PCO and maybe prevent it,” he continues. “I do think that a lot of IOL manufacturers have provided lenses that have that very sharp, right angle of the posterior optic edge on the IOL, and that can help to prevent or slow down PCO.”
Maybe the addition of a sharp edge on an IOL can help, but this all circles back to a point that Dr. McKinney made earlier. If these IOLs can make the lens capsule “shrink wrap” around the posterior capsule, as Dr. Mamalis suggested, then these lenses give you a better chance of success with an earlier Nd:YAG capsulotomy, alluded to by Dr. McKinney. In the end, the IOL of choice will have some impact over how PCO is developed and when a capsulotomy can be performed.
Though YAG capsulotomy has become a time-honored skill, surgeons say it still should be approached with care. “However, while YAG capsulotomy can be performed in a fairly short period of time by a skilled ophthalmologist, it’s not easy and it’s not risk-free,” Dr. McKinney notes. “It has the potential to do permanent damage to the eye if it’s done incorrectly. So, for that reason, it should only be performed by ophthalmologists who’ve had hundreds of hours of surgical training in the care of actual patients."
Drs. McKinney and Mamalis have no related financial disclosures.
1. Ursell PG, Dhariwal M, O’Boyle D, et al. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: A real-world evidence study of 20,763 eyes. Eye (Lond) 2020;34:5:960-968.
2. Hayashi H, Hayashi K, Nakao F, et al. Elapsed time for capsular apposition to intraocular lens after cataract surgery. Ophthalmology 2002;109:8:1427-31.
3. Sanders DR, Kraff MC, Lieberman HL, et al. Breakdown and reestablishment of blood-aqueous barrier with implant surgery. Arch Ophthalmology 1982;100:4:588-590.
4. Ferguson VM, Spalton DJ. Recovery of the blood-aqueous barrier after cataract surgery. Br J Ophthalmology 1991;75:2:106-10.
5. Gautam AK, Nath R, Kumar D, et al. Early re-establishment of blood aqueous barrier after phacoemulsification. India J Ophthalmology 1998;46:3:173-174.
6. Gu X, Chen X, Jin G, et al. Early-onset posterior capsule opacification: Incidence, severity and risk factors. Ophthalmology Therapy 2022;11:1:113-123.
7. Congdon N, Fan H, Choi K, et al. Impact of posterior subcapsular opacification on vision and visual function among subjects undergoing cataracts surgery in rural China: Study of Cataract Outcomes and Up-Take of Services (SCOUTS) in the Carin is Hip Project, report 5. Br J Ophthalmology 2008;92:5:598-603.
8. Grzybowski A, Kanclerz P. Does Nd:YAG capsulotomy increase the risk of retinal detachment? Asia-Pacific J Ophthalmology 2018;7:5:339-344.
9. Lee C-Y, Lu T-T, Meir Y-JJ, et al. Refractive changes following premature posterior capsulotomy using neodymium: yttrium-aluminum-garnet laser. J Personal Medicine 2022:12:2:272.
10. Chen H-C, Lee C-Y, Liu C-F, et al. Corneal endothelial changes following early capsulotomy using neodymium: yttrium-aluminum-garnet laser. Diagnostics 2022;12:1:150.
11. Bhargava R, Kumar P, Sharma SK, et al. Peeling and aspiration of Elschnig pearls! An effective alternative to Nd:YAG laser capsulotomy! Indian J Ophthalmology 2013;61:9:518-20.