Researchers at the University of California-Irvine are working with a femtosecond laser that doesn't cut tissue. Instead, it uses special properties of light to allow researchers to view structures that normally can only be viewed after sectioning them surgically, including the lamina cribrosa. Though the device still has to be perfected before it's ready to be commercialized, its users say it gives them unique views of the eye.
The laser provides images through an optics principle known as second-harmonics. "When you focus two photons on a linear molecule that's got polarity to it, one photon is absorbed and one is released," says Don Brown, PhD, assistant professor in the ophthalmology department at UC-Irvine. "And the one that's released [which is captured by the imager] is half the wavelength of those going in. So, you can essentially use a femtosecond laser to deliver packets of photons at longer wavelengths that interact with the collagen in the tissue, but release photons at half that wavelength. It's basically a mechanism to visualize extracellular matrix components without having to stain, fix or section tissue." Currently, due to the energies involved, it can't be used in live tissue, but can be used in fresh tissue without staining or sectioning. "As you go up above 10 mW or so of power going in you start causing tissue damage, which you obviously don't want for imaging," says Dr. Brown. "Right now, I'm at around 20 mW or so, so I have to reduce it by a factor of two. The other issue is going to be the optics and acquisition of the tissue image. But certainly, as an ex vivo technology, it's really quite nice, and basically non-destructive because you use focal sectioning rather than actual sectioning."

Researchers are using a femtosecond laser to image the details of the optic nerve head.
The setup for capturing the returning image is similar to the microscope system used for fluorescent imaging: a photomultiplier in a collection tube. It projects 800-nm light and collects the 400-nm light that comes out.
Dr. Brown's group is interested in using the laser to examine biomechanical structural components of the eye, especially with regard to glaucoma and the lamina cribrosa. He recently published a paper on his work in the Journal of Biomedical Optics.1
In the paper, Dr. Brown and colleagues were able to visualize the lamina cribrosa of an eye-bank eye without having to section it. They then hooked the eye to an artificial chamber that allowed them to alter the intraocular pressure and view the effects on the lamina cribrosa. "In essence, the lamina cribrosa forms a network of woven collagen," explains Dr. Brown. "There are, for lack of a better word, pores present there at the point where the axons for the nerves leave the eye. When you increase pressure, we saw that, just like applying pressure to a balloon with a hole in it, the majority of the pores at the lamina increased in size, like the hole in the balloon would. However, there was a difference when you got near the periphery of the opening—those pores tended to collapse with an increase in pressure. This is reasonably consistent with the known effects of glaucoma: You tend to lose peripheral vision first, and those peripheral axons tend to exit the eye through the periphery of the optic nerve head, so we may have been seeing the effects of an acute rise in pressure."
Dr. Brown's colleague James Jester, PhD, presented a paper at the 2007 annual meeting of the Association for Research in Vision and Ophthalmology using fresh tissue from surgical specimens or eye-bank eyes and femtosecond-generated second-harmonic signals to look at the collagen organization in the normal adult cornea and corneas with keratoconus. (Jester J. IOVS 2007;48: ARVO E-abstract 8.) "Transmission electron microscopy can identify corneal lamellae, but you can only see a small region, a thin section, with that," says Dr. Jester. "With this technology, you can now define the lamellae and see how they're organized three dimensionally, though at a lower resolution. We've found that the lamellae are sort of orthogonally oriented lamellae that insert in Bowman's layer. Then when we looked at keratoconus, we found that these orthogonally arranged, or what we called sutural lamellae, are absent. This suggests that these lamellae are a site of action for keratoconus. Either they're destroyed during the process of the disease or they're not formed during corneal development."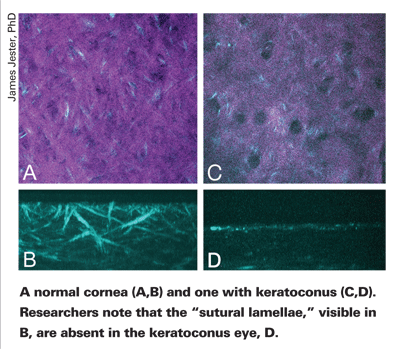
Dr. Jester says the ultimate goal with femtosecond imaging is laser-induced optical breakdown, which would involve using the same femtosecond laser to both cut tissue and then instantly image it to evaluate its healing and the effects of the cut. "That's what we're moving toward," he says.
1. Brown DJ, Morishige N, Neekhra A, Minckler DS, Jester JV. Application of second harmonic imaging microscopy to assess structural changes in optic nerve head structure ex vivo. J Biomed Opt. 2007;12:2:024029.



