Meibomian gland dysfunction is a relatively common affliction, and may present in upwards of 60 percent of dry-eye sufferers.1 It's well-accepted that meibomitis is characterized by abnormal meibum secretions, differing from normal secretions in viscosity and/or color as well as physical composition. However, meibomitis's etiology remains unknown, there's no universal definition or classification for it, and it has no proven treatment. Many theories exist as to its causes, though most studies haven't been replicated and have used different scales and methodologies. As many ideas are in debate, a multidisciplinary approach to understanding meibomian gland dysfunction and disease is merited. Given that the meibomian gland is a modified sebaceous gland, the intricate interlacings of human biological systems suggest that an interdisciplinary perspective of gland dysfunction in related fields could benefit ophthalmology.
This month's column will investigate the potential benefits of cross-specialty collaboration toward a more universal understanding of MGD. First, we'll compare and contrast the anatomy, physiology and pathophysiology of sebaceous and meibomian glands. Then, we'll outline some theories and examine several future interdisciplinary endeavors in order to get a better understanding of the disease and identify new treatment options.
Gland Anatomy and Physiology
Sebaceous glands are found in all mammals except for whales and porpoises and almost everywhere on the human body, with the exception of the palms of the hands and soles of the feet. Sebaceous glands are innumerable on the human body, with 400 to 900 glands/cm2 on the face alone.2 The majority of these glands form a pilosebaceous unit with their associated hair follicle and are responsible for the release of sebum, an oily secretion. By definition, the sebaceous gland is a branched alveolar holocrine gland, meaning that the excretion (sebum) results from the growth and subsequent bursting of mature alveolar cells. Pilosebaceous glands open into the piliary canal, while free sebaceous glands excrete their contents directly onto the skin surface.2 In non-human mammals the sebum prevents heat loss and functions as a sexual attractant and territorial marker. Human sebum functions are theorized to include establishing and maintaining a protective lipid film over epithelial surfaces, retardation of water evaporation, protective shielding against bacterial and fungal infections and delivery of antioxidants and pheromones.3,4
|
• Lipids. Sebum composition includes triglycerides, free fatty acid, wax esters, squalene and cholesterol. By weight, sebum is 57 percent triglycerides, diglycerides and free fatty acids (27 percent saturated, 68 percent unsaturated); 25 to 26 percent wax esters; 12 to 15 percent squalene; and 2 to 3 percent cholesterol.4,8 One of the most prominent distinctions of human sebum is the level of squalene secreted that isn't converted to cholesterol; analysis of sebum from other mammals reveals a level that ranges from zero squalene in mice and rabbits to 0.5 to 1.5 percent in rats. In contrast, cholesterol levels in non-human mammalian sebum (by weight) were as high as 13 percent in mice. While the causes and implications of this distinction are unknown, theories suggest over-expression of squalene synthase, environmental influences and decreased activity of enzymes responsible for squalene conversion into cholesterol may be factors.4 Research into the properties and functions of sebum is ongoing.
Like sebaceous lipids, meibomian lipids are synthesized via breakdown of the acinar cells. In doing so, the glands produce both polar and non-polar lipids, but release them all together as a substance called meibum. There are more than 100 lipid components of meibum which serve many functions. One literature review describes ranges of normal human meibum lipids as follows:
• 1 to 38 percent hydrocarbon;
• 13 to 68 percent wax ester;
• 8 to 39 percent sterol ester;
• 2 to 18 percent diesters;
• 2 to 43 percent triglycerol;
• 0 to 5 percent alcohols;
• 0 to 24 percent free fatty acids;
• 0 to 1 percent cholesterol; and
• 0 to 16 percent polar lipids.9
Together, these lipids maintain the smooth optical surface required for optimal visual acuity, reduce evaporation of the tear film, lower tear film surface tension to increase stability, prevent tear spillover to the lid margin, and seal the lid margins during sleep.5,7
Of particular importance in dry-eye research is the role these lipids play in tear-film integrity and maintenance. The tear film is composed of three major components: aqueous; mucin; and lipid. The lipid layer is the product of meibomian secretions and is made up of two layers: the non-polar, air-tear film interface (that prevents evaporation to the environment) and the polar, lipid-aqueous interface (that stabilizes the overall lipid layer).6 The organization of the non-polar lipids (e.g., wax and cholesterol esters and triglycerides) that retard evaporation is defined by the location of the polar lipids (e.g., phospholipids and sphingomyelin) beneath them; the outer layer depends structurally on the support of the inner sub-layer.6 This structure is also the basis for the "wrinkle and fold" phenomenon that occurs with each blink.7 If not for the polar/non-polar interaction, the lipid sub-layers could mix and their protective abilities could be lost, since uneven spreading of the tear film can lead to evaporation and decreased tear-film breakup time.
Gland Pathophysiology
Gland disorders can take the following forms:
• Acne vulgaris. This is the most common disorder involving the sebaceous gland; it is estimated to occur in 80 percent of adolescents during puberty. Sebogenesis escalates with the increasing amounts of androgens characteristic of puberty, and thus acne development typically coincides with the pubescent period. More specifically, acne develops via colonization of the hair follicle by P. acnes (nourished by sebum), hyperkeratinization of the upper follicle, and release of inflammatory mediators into the skin.4
Treatment approaches may vary, but generally consist of topical retinoids, antimicrobials, keratolytics, anti-inflammatories, oral hormones (e.g., steroids, contraceptives) or some combination of these.10
|
• Seborrheic dermatitis. This is a chronic inflammatory condition of the skin that's also associated with the sebaceous glands. It presents as red, scaly patches and is more commonly found in areas with a dense distribution of sebaceous glands (e.g., the face and scalp).11 Seborrheic dermatitis also manifests in up to 70 percent of infants, generally affecting the scalp (where it is called "cradle cap"), the face and the diaper area, but the condition typically resolves by 1 year of age.11 While the role of the sebaceous glands is unclear, seborrheic dermatitis is more common during periods of increased sebum production, and seborrhea may predispose patients to seborrheic dermatitis.4,11
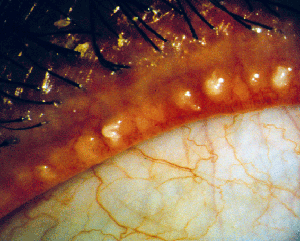
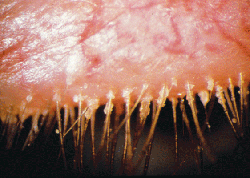
• Meibomian gland dysfunction. Anatomical differences between normal and MGD patients can be observed with transillumination of the lid margin: Normals show grape-like clusters of acini forming long meibomian glands while MGD patients are marked by a loss of glands, dilation of the central duct and other cystic changes. Lipid profiles help clinicians distinguish between the types of MGD responsible.6
Furthermore, MGD patients have been said to present with hyperkeratinization of the central duct, in which excess keratin causes adherence of sloughed epithelial cells and blockage of the gland.
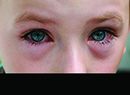
MGD patients may also have congenitally absent or deficient glands, or experience gland dropout (loss of gland function) as well as enlargement or laxity of the glands with age.5,7 MGD patients also exhibit dry-eye symptoms and chalazia. The latter seem to replace meibomian glands, so it's generally believed that they arise as a result of obstruction.5 Researchers at Ora Inc. developed meibomitis scales to track these glands and their activity via characterization of the physical appearance of meibum, vascularity surrounding the glands and the number and morphology of the functional glands. While no therapy is currently approved for meibomitis, clinical practice includes off-label use of antimicrobials, warm compresses and lid scrubs.
Dividing meibomitis into several disease categories seems to be typical. Some break it down to two categories: obstructive and seborrheic.6 Other studies have classified six or more variations.5 Researchers are also interested in quantifying the amount of meibum secreted and the recovery time (Shapiro A, et al. IOVS 2008;49:ARVO E-abstract 84; Blackie CA, et al. IOVS 2008;49:ARVO E-abstract 86). Transillumination of the lid margin to show anatomical differences between normal and MGD patients seems to be common.6,12,13 Researchers have pointed to myriad causes of meibomitis, including hormonal deficiency,7,14,15 the influence of a neural feedback loop,6,16 bacterial colonization17,18 and the use of contact lenses.19,20
• Hormone deficiency. Sebaceous gland activity is largely under endocrinologic control.2 During childhood, the sebaceous glands are relatively inactive until the teenage years, at which point they increase in size and secrete larger quantities of sebum. Sebum levels stay relatively constant until about 80 years of age in males and until menopause in females.3 Both the increase in gland activity accompanying puberty and the decrease observed later in life are attributed to changes in androgen production.
Like sebaceous glands, meibomian glands are driven in part by hormone levels. Male patients un-dergoing anti-androgen therapy for prostate disease have demonstrated increased rose bengal and fluorescein ocular surface staining,14 as well as decreasing di- and triglyceride, wax ester and cholesterol ester concentrations, while increasing free cholesterol content.14,15 Females with androgen insensitivity syndrome and individuals of either gender with Sjögren's syndrome show a general decrease in total lipid formation.14,15 This leads to dry-eye symptomatology.7
• Neuronal influence. Initially, sebaceous glands were thought to lack innervation until research emerged demonstrating the presence of nerve fibers within the sebaceous lobule. The function of these fibers remains largely unclear, although there is evidence that increased innervation and increased expression of nerve growth factor occurs with acne.21
Unlike the vague descriptions of innervation related to typical sebaceous glands, the meibomian gland is known for its ample innervation. Meibomian gland acini are associated with parasympathetic, sympathetic and sensory nerve sources alike; and the vessels surrounding the meibomian glands are richly innervated as well.6
The meibomian and lacrimal glands seem to have vasoactive intestinal polypeptide (VIP) nerves in communication with the acini cells. The name is somewhat of a misnomer: VIPs are found from the gastrointestinal tract to the brain, and the brain generally coordinates intercellular communication. Therefore, the two glands may operate in tandem under the influence of the same neurotransmitters.6
Other neuropeptides include substance P (which is important in the function of pain reception) and calcitonin gene-related peptide (a vasodilator as well as a pain transmitter), among others.7, 22
Sebum and Meibum in Defense
It's not a new discovery that the skin and eye contain self-disinfecting properties, or that these are attributable to lipids. Myriad antimicrobial lipids coat the epidermis and conjunctiva alike. In the skin, lipid is packed in lamellar granules, the contents of which are exocytosed into the intercellular spaces of the epithelium.17 Thus, many phospholipids are embedded in the lipid matrix of the skin. The phospholipids known as triglycerides also undergo hydrolysis to create free fatty acids;17epidermal acid lipase is largely responsible for this hydrolysis, which yields sapenic and lauric acid, both of which have demonstrated antibacterial properties. Many organisms are susceptible to these acids, such as S. aureus, S. pyogenes, S. epidermidis and Micrococcus, but probably the most noteworthy is the susceptibility of methicillin-resistant S. aureus, or MRSA.17
The epidermis maintains many antimicrobial peptides near potential points of entry (e.g., around hair follicles) and damage to the epithelium incites a rapid increase in their production.17 This, in turn, acts as a chemoattractant for leukocyte recruitment. On the ocular surface, fatty acids act in synergy with tear lactoferrin and lysozyme. However, the implications of deficiency in the quality or quantity of meibomian secretions may inhibit the innate defense mechanism attributed to these antimicrobial capabilities, putting the lid margin at greater risk for microbial invasion and potentially resulting in the development of meibomitis. The addition of fatty acids to the ingredients of antimicrobial prophylactic agents has been suggested by at least one investigator.17
Furthermore, the bacteriological origin of sebaceous gland pathology may suggest a need for consideration of possible bacteriological mechanisms in meibomitis. Quorum sensing, a bacterial communication system involving altered expression of genes depending on bacterial populations, is a potential mechanism for the development of pathological bacteria from normal flora.23 Certainly, research demonstrating altered flora on the eyelids of patients with meibomitis compared to normals suggests that the use of antimicrobial and/or anti-inflammatory agents to treat the disease could be beneficial, or at least should be an avenue for further investigation.
Future Research
Interdisciplinary application of the theories regarding sebaceous glands, lipids and meibomian gland pathophysiology could bring substantial benefits in the exploration of new treatment approaches to meibomitis. A Special Interest Group submitted to the 2010 Annual Meeting of the Association for Research in Vision and Ophthalmology aims to address the challenge of treating meibomitis through the collaboration of a team of researchers and clinicians specializing in ophthalmology, sebaceous lipids, meibomian glands and quorum sensing. If accepted, the Special Interest Group's presentations and discussion will offer the potential for enhanced understanding of the mechanisms of meibomitis, and may suggest new or revised avenues for therapeutic success. REVIEW
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Ms. Howe and Ms. Maffei are medical writers, and Mr. Shapiro is director of anti-infectives and anti-inflammatories at Ora Inc. in Andover.
1. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol 1995;113:10:1266-70.
2. Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev 1989;69:2:383-416.
3. Pochi PE, Strauss JS, Downing DT. Age-related changes in sebaceous gland activity. J Invest Dermatol 1979;73:1:108-11.
4. Smith KR, Thiboutot DM. Thematic review series: Skin lipids. Sebaceous gland lipids: Friend or foe? J Lipid Res 2008;49:2:271-81.
5. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond) 1991;5(Pt. 4):395-411.
6. McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf 2003;1:3:97-106.
7. Mathers WD. Meibomian Gland Disease. New York: Basel, 1991:247-67.
8. Wertz PW. Sebum secretion and acne. In: Webster GF, Rawlings, A.V., eds. Acne and its therapy. New York: Informa healthcare, 2007:37-44.
9. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids--A review. Curr Eye Res 2008;33:5:405-20.
10. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol 2009;60(5 Suppl):S1-50.
11. Naldi L, Rebora A. Clinical practice. Seborrheic dermatitis. N Engl J Med 2009;360:4:387-96.
12. Robin JB, Jester JV, Nobe J, et al. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction.A clinical study. Ophthalmology 1985;92:10:1423-6.
13.Shimazaki J, Goto E, Ono M, et al. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology 1998;105:8:1485-8.
14. Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab 2000;85:12:4874-82.
15. Sullivan DA, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci 2000;41:12:3732-42.
16. Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 2004;78:3:409-16.
17. Drake DR , Brogden KA, Dawson DV, Wertz PW. Thematic review series: Skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res 2008;49:1:4-11.
18. Suzuki T, Mitsuishi Y, Sano Y, et al. Phlyctenular keratitis associated with meibomitis in young patients. Am J Ophthalmol 2005;140:1:77-82.
19. Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009;116:3:379-84.
20. Ong BL. Relation between contact lens wear and Meibomian gland dysfunction. Optom Vis Sci 1996;73:3:208-10.
21. Knaggs H. Cell biology of the pilosebaceous unit. In: Webster GF, Rawlings A.V., eds. Acne and its therapy. New York: Informa healthcare, 2007:9-36.
22. McCulley JP, Shine WE. The lipid layer of tears: Dependent on meibomian gland function. Exp Eye Res 2004;78:3:361-5.
23. Keller L, Surette MG. Communication in bacteria: An ecological and evolutionary perspective. Nat Rev Microbiol 2006;4:4:249-58.