Since dry eye is a common ophthalmic complaint, it’s been a popular target for drug and even device makers in recent years. Currently, many companies are working to develop additional therapeutic agents to treat this population, with many of these agents having unique and novel mechanisms of action. Here are some products you may have the option of incorporating into the dry-eye treatment protocol at your practice in the coming months or years.
Reproxalap (Aldeyra)
Aldeyra has been investigating a novel agent for the treatment of dry eye, reproxalap, which is a small-molecule reactive aldehyde species (RASP) inhibitor. The drug fights ocular inflammation and improves dry-eye symptoms through a unique mechanism of action: while RASP molecules bind to cellular biomolecules, disrupting their function and activating pro-inflammatory mediators, reproxalap inhibits this inflammation by binding the free aldehydes and reducing RASP levels as a result.
In the second quarter of 2021, results were published for the Phase IIb trial studying the effectiveness of reproxalap in reducing dry-eye signs and symptoms. The company says the agent demonstrated rapid, broad and clinically relevant symptomatic control in a cohort of 300 DED patients over 12 weeks of therapy. It also showed significantly greater improvement in signs of the disease vs. the vehicle, as demonstrated by fluorescein staining.1 Patients in the study reported the drops to be highly tolerable.
“We’re learning so many different pathways for upregulation and control of the inflammatory response,” says John Sheppard, MD, who practices at and is president of Virginia Eye Consultants and is a professor of ophthalmology at Eastern Virginia Medical School in Norfolk. “Aldehydes are very important in inflammation; The aldehyde species has is a wide variety of tissue-destructive and cytokine-activating functions that are misdirected in autoimmune disease or inflammatory processes of aging. It turns out dry-eye patients have high levels of a byproduct of aldehyde metabolism: malonaldehyde. If you put a RASP-inhibitor drop, such as reproxalap, in the eye of a dry-eye patient, the decrease in that metabolite, malonaldehyde, corresponds to improvement in dry-eye signs and symptoms.”
Results were published last month from another Phase II clinical trial that compared ocular discomfort and itching symptom scores of reproxalap versus Xiidra in 56 patients with DED. They found that both patient-reported ocular discomfort (p=0.002) and itching (p=0.01) were statistically lower with reproxalap than with Xiidra.
Aldeyra is currently finishing Phase III trials to further evaluate the drug.
 |
| Researchers are working on a variety of new ways to manage the symptoms and signs, such as corneal staining, of dry eye. |
RGN-259 (RegeneRx)
RegeneRx has conducted a series of Phase III clinical trials since 2015 for its Tß4-based sterile and preservative-free eye drop, RGN-259, which has been approved for the treatment of neurotrophic keratitis in the United States since 2013. The drop’s active ingredient, Tß4, promotes corneal and ocular surface healing by facilitating epithelial cell migration and increasing cell-cell and cell-matrix contacts, which in turn reduces apoptosis and inflammation in the cornea.
The company announced in March 2021 that the most recent clinical trial completed, ARISE-3, which enrolled 700 participants, didn’t meet its primary outcome measures. Still, the results demonstrated improvement in ocular grittiness at one and two weeks after treatment that was statistically significant, according to the company. RegeneRx notes that in all three clinical trials conducted so far (ARISE-1, -2 and -3), results have shown an improvement in various signs and symptoms of dry-eye syndrome, and the drug has proven safe.
The company says it will continue to analyze the clinical data and investigate the efficacy of RGN-259 and that it’s looking towards a pre-Biologics License Application (BLA) meeting with the FDA in the near future.
Visomitin (Mitotech)
Visomitin is a solution of SkQ1, a novel mitochondrial-targeted antioxidant currently being investigated in the treatment of various inflammatory ocular surface conditions including moderate to severe dry eye.
“The mechanism of action includes the inhibition of inflammatory breakdown products and mitochondrial metabolism,” says Dr. Sheppard. “This produces different types of effects including anti-inflammatory, anti-tear secretory deficiency and a regenerative effect on the lacrimal accessory gland tissue rejuvenation process, which may, although this is not yet documented, apply to other sources of tears such as meibomian glands, accessory lacrimal glands and goblet cells. It also has a direct effect on epithelial repair and healing.”
Results from VISTA-1, a Phase IIb/III clinical study that enrolled 450 patients, found that relative to the vehicle, SkQ1 demonstrated a statistically significant reduction of ocular discomfort by week four while maintaining an excellent safety profile and tolerability similar to that of an artificial tear. The more recent VISTA-2 Phase III trial, including 610 patients, had similar outcomes; both VISTA-1 and VISTA-2 demonstrated statistically significant improvement in conjunctival fluorescein staining vs. vehicle and improvement of best-corrected visual acuity at week four.
“Patients who have failed other medications indicated for dry eye may be great candidates for this new therapy because of the very new and novel mechanism of targeting mitochondrial metabolism,” Dr. Sheppard says. He notes that one unique aspect of this drug is that it “does more than fight inflammation because it targets mitochondria that present in all cell types on the ocular surface, from the cornea, conjunctiva and secretory apparatus to the lid and cutaneous tissues adjacent to the lid.”
The last trials designed to confirm these outcomes, VISTA-3 and VISTA-4, are scheduled to begin in the second half of 2022.
CyclASol (Novaliq)
CyclASol is an anti-inflammatory solution containing 0.1% cyclosporine A in a unique forumla developed to treat DED. The company says the therapeutic approach involves the company-designed water-free technology, EyeSol, which is meant to increase the time the drug resides on the ocular surface and enhance bioavailability to target tissues, allowing the cyclosporine A to work more quickly and with maximum efficacy.
“The semifluorinated alkane molecules in EyeSol are water-free, don’t require preservatives and are able to solubilize a wide variety of active pharmaceutical ingredients, some of which aren’t very soluble in other vehicles,” says Dr. Sheppard. “Also, the semifluorinated alkanes have unique molecular characteristics; they create a drop that’s only 20 µl in size, much smaller than a standard solution or suspension, which is about 50 µl. Considering the tear film only holds about 20 µl to 30 µl in the cul-de-sac and on the surface of the eye, this medication won’t spill over on the cheek like virtually all other topical medications.”
The drop is in late-stage development after achieving positive results in three completed clinical trials. In its Phase IIb/III ESSENCE-1 trial including 328 patients with DED who were treated for 12 weeks, researchers found CyclASol to be superior to vehicle in total corneal fluorescein staining at week four. After two weeks, this difference was already statistically significant and remained so throughout the study. The treatment was also shown to be safe and well-tolerated.2
Results of the latest trial in Phase III, ESSENCE-2, were just released by the company in December 2021. Designed to replicate and confirm the findings from ESSENCE-1, the study enrolled a larger cohort of 834 DED patients and found once again that improvement of total corneal fluorescein staining was achieved at week four. By this time point, nearly three in four patients (71.6 percent) improved by at least three grades in total corneal staining. Compared to vehicle-treated patients, those receiving CyclASol demonstrated statistically significant improvements across a range of common symptoms.
The company plans to conclude the drug’s clinical development program with a multicenter, 12-month safety extension trial (ESSENCE-2 OLE), which completed enrollment in the summer of 2021 and includes 202 patients.
NOV03 (Bausch + Lomb)
Another treatment in the pipeline that uses EyeSol technology is NOV03 (perfluorohexyloctane). This drop was specifically developed for patients with meibomian gland dysfunction contributing to their dry eye, which studies have indicated is the case for a majority of DED patients.3
“If approved, NOV03 may be the first pharmaceutical therapy in the United States indicated for this patient population,” says Joseph Tauber, MD, founder of Tauber Eye Center in Kansas City, Missouri.
The first Phase III trial, GOBI, enrolled 597 participants, half of which administered the drop twice a day and half of which administered it four times a day. The company reported that patients on either dosing regimen demonstrated an improvement in total corneal fluorescein staining and eye dryness (subjectively scored by the patient) vs. the control at eight weeks.
Midway through 2021, the company completed the latest Phase III trial, MOJAVE, which included 620 participants who received either NOV03 or a saline solution (the placebo). Both studies were able to meet their primary endpoints for reduction of the signs and symptoms of DED compared to the control saline groups.
Dr. Tauber notes that trials from the second phase had similar results: “In the Phase II clinical trial, SEECASE, the effect of improved total corneal fluorescein staining started as early as two weeks after start of treatment and was maintained over the entire duration of the trial for both treatment regimens (two and four times daily).”
Bausch + Lomb expects to conclude its investigation of NOV03 with the current ongoing 12-month safety extension trial, KALAHARI. The company anticipates filing a New Drug Application in the first half of this year.
Lacripep (Tear Solutions)
This therapeutic drop was developed to treat symptoms of dry eye and primary Sjögren’s Syndrome through the use of a proprietary synthetic fragment of lacritin, a natural tear protein that’s limited in tears of people with dry eye. The company says the solution is water-soluble and designed to work by restoring basal tearing and homeostasis of the ocular surface without causing irritation.
The Phase II, double-masked study involved 204 participants with dry eye caused by primary Sjögren’s receiving either 0.01% Lacripep, 0.005% Lacripep or a placebo solution, the second of which appeared to perform best among the cohort. Trial results were presented at ARVO 2021, one of the outcomes being that patients with moderate-to-severe dry-eye symptoms using the 0.005% concentration showed significant improvement in inferior corneal fluorescein staining and reduced levels of burning and stinging after two weeks, although the latter wasn’t significant at four weeks.
The company says it will continue conducting clinical studies to investigate the safety and efficacy of the drop, including its optimal dose and duration of administration.
Another protein therapy in the dry-eye pipeline is ECF843 (Novartis), which is in Phase II clinical trials. In a study of 718 participants, the recombinant human lubricin (rh-Lubricin) protein has shown its ability to provide immediate symptom improvement likely by increasing lubrication across the ocular surface. The company says the drug is hypothesized to restore the function of the tear film, reduce friction and alleviate dry-eye signs and symptoms.
HBM9036 (Harbour BioMed)
This drug, with the proposed brand name Tanfanercept, is a tumor necrosis factor receptor 1 fragment. The mechanism of action involves the binding and blocking of TNF-α, which results in suppressed inflammation after drop use. The company released results from Phase II trials in 2020, which cited that Tanfanercept (0.25%) demonstrated significant improvements in signs with a good safety profile and rapid onset. The Phase III clinical trial began in March 2021, and the company expects to submit a BLA application to the FDA in 2022.
AR-15512 (Aerie Pharmaceuticals)
Investigational eye drop AR-15512 was formulated to tackle the signs and symptoms of DED through its active ingredient: a proprietary agonist of transient receptor potential melastatin 8 (TRPM8) cold thermoreceptor, which creates a comfortable cooling sensation across the eye, according to the company. Aerie explains that TRPM8 channels are cold-sensitive, found on the eyelid and cornea and play a role in helping the tear film maintain homeostasis. Increased TRPM8 activity can lead to increased tear production and blink rate, while its dysfunction may cause or worsen dry eye.
In the fall of 2021, Aerie released the Phase IIb (COMET-1) clinical trial results for AR-15512, which enrolled 369 dry-eye patients. Over an 84-day period, the drop showed and maintained significant improvement in both symptoms and signs, which was observed as early as week two. The company notes that efficacy was observed after the first dose and that the drop appeared to be safe and well-tolerated in both concentration levels tested (0.0014% and 0.003%).
Aerie reports that it plans to meet with the FDA in the first quarter of this year to discuss the results of Phase II and to begin planning the two anticipated Phase III efficacy studies, each lasting three months, as well as a final safety study.
AZR-MD-001 (Azura Ophthalmics)
This keratolytic drop treats MGD and evaporative DED. The Phase IIb study evaluated the drug’s safety and efficacy in treating 95 patients with MGD and found there was a statistically significant and clinically meaningful reduction of symptoms by the end of the three-month trial period in patients receiving the 0.5% or 1% concentration. More than half of the patients (58 percent) were asymptomatic after the three months, compared to 16 percent of patients in the control group. They also observed that in both concentration groups compared with controls, the number of glands secreting meibum showed significant improvements in as little as one month.
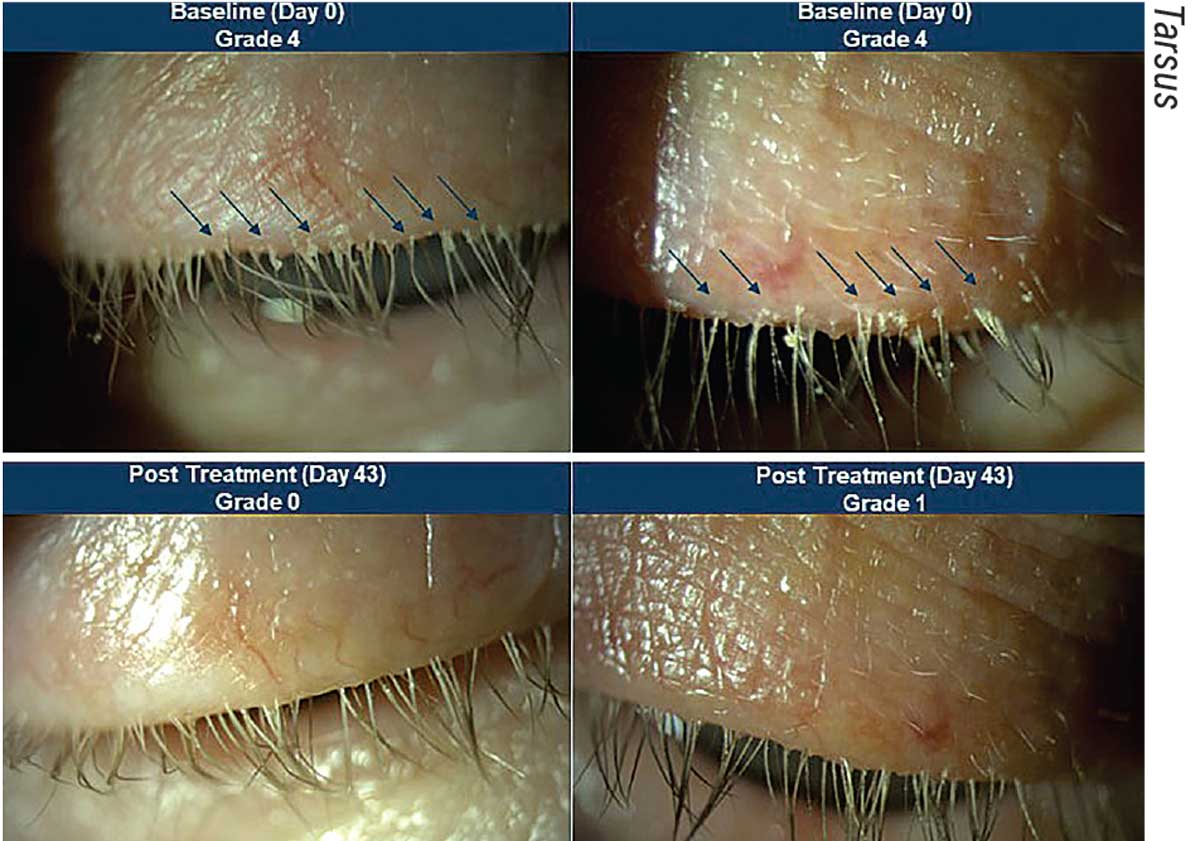 |
| Before and after images of a patient treated with TP-03 in the Saturn 1 Phase IIb/III trials. The top two photos show Demodex blepharitis in a patient at day zero, which looks to have improved significantly by day 43. |
TP-03 (Tarsus)
TP-03 is currently undergoing trials as a novel treatment for Demodex blepharitis, a common type of blepharitis caused by an infestation of Demodex mites within the eyelash follicles. TP-03 is formulated with lotilaner, an anti-parasitic agent that works by paralyzing and eradicating Demodex mites through selectively inhibiting parasite-specific GABA-Cl channels.
“It’s a potent, non-competitive antagonist of insect and arachnid GABA-Cl channels and a highly lipophilic molecule, which may promote its uptake in the oily sebum of the hair follicle where the mites reside,” says Elizabeth Yeu, MD, a partner and practicing ophthalmologist at Virginia Eye Consultants and medical director of the Virginia Surgery Center. “Essentially, the treatment is able to target and kill the mites while being safe for use on humans.”
The company released results last June from the Phase IIb/III Saturn-1 pivotal trial including 421 Demodex patients who had more than 10 collarettes on the upper lid, at least mild erythema of the upper eyelid margin and at least 1.5 mites per lash on the upper and lower eyelids combined. Tarsus reports that TP-03 met both the primary and secondary endpoints and was well-tolerated. They note that 81 percent of patients on TP-03 achieved a clinically meaningful collarette cure (10 or fewer collarettes, grade 0 or 1) compared with 23 percent of patients who received the vehicle. They also report that 68 percent of patients on TP-03 achieved mite eradication (defined as a mite density of zero mites per lash) by day 43 compared with 18 percent for the vehicle.
“One in five patients experienced a complete erythema cure and 45 percent showed at least a one-grade improvement,” says Dr. Yeu. “Both results were statistically significant compared to vehicle, and very meaningful to me as a clinician and for my patients.”
TP-03 is currently being evaluated in the Phase III pivotal trial (Saturn-2) with the same primary endpoints. Results are anticipated to be released during the first quarter of this year. If the data is positive and similar to that from Saturn-1, the company plans to submit a New Drug Application this year.
ST-100 (Stuart Therapeutics)
At the beginning of this year, Stuart Therapeutics announced the Phase II trial results for its investigational drop for dry eye, ST-100. The 160-patient study found that the drug met the Schirmer’s Responder Rate endpoint at four weeks (defined based on FDA guidance as a statistically significant difference in the percentage of patients achieving a 10 mm or greater increase in Schirmer’s tear test scores). As early as day seven, ST-100 showed significant results in several symptoms and ocular surface staining scores, the company says.
Clinical trials will soon evolve to Phase III, the company says.
SURF-100 and SURF-200 (Surface Ophthalmics)
Surface Ophthalmics is developing two therapeutic drops for sufferers of dry eye, both of which use the company’s patented Klarity diluent as the vehicle: SURF-100 (mycophenolate sodium and betamethasone sodium phosphate), made for the treatment of chronic DED; and SURF-200 (betamethasone), which treats acute/episodic dry eye.
The two products are currently in Phase II clinical studies. The trial for SURF-100 is enrolling about 300 patients, and the trial for SURF-200 is enrolling between 120 and 140 patients. In the first two months of 2021, the company reported that the first patients in both trials had been dosed.
GLK-301 (Glaukos)
Rather than an eye drop, this topical cream, code-named GLK-301, is applied to the outer eyelid. The active ingredient, pilocarpine, is intended to help relieve DED signs and symptoms. The company announced last month that it had begun enrollment for the Phase II clinical trial, which will include 200 patients with DED, including 20 with Sjögren’s. The trial is meant to evaluate the safety and efficacy of GLK-301 applied twice daily to lids over four weeks, followed by a two-week safety follow-up period. Three dose levels of GLK-301 will be tested and compared with placebo.
Dr. Sheppard is a consultant for Aldeyra and Bausch + Lomb. He has been involved in clinical research for Novartis, Novaliq, Aldeyra, Bausch + Lomb and Tear Solutions. He is also on the advisory board for Mitotech, Aldeyra, Bausch + Lomb, Novartis and Novaliq and is on the Speakers Bureau at Bausch + Lomb.
Dr. Yeu is the chief medical advisor for Tarsus and also serves on their Board of Directors.
Dr. Tauber consults for Bausch + Lomb and Novaliq.
1. Clark D, Tauber J, Sheppard J, Brady TC. Early onset and broad activity of reproxalap in a randomized, double-masked, vehicle-controlled phase 2b trial in dry eye disease. Am J Ophthalmol 2021;226:22-31.
2. Sheppard JD, Wirta DL, McLaurin E, et al. A water-free 0.1% cyclosporine a solution for treatment of dry eye disease: results of the randomized chase 2B/3 ESSENCE study. Cornea 2021;40:10:1290-1297.
3. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:3:276-283.