Over the past decade, there have been major advances in the field of intraoperative optical coherence tomography. From the development of the portable OCT probe to the first microscope-integrated system, intraoperative OCT has evolved from a research idea to a clinically viable technology. Advances such as heads-up visualization and microscope-integration, as well as the results from large clinical studies, have pushed the field forward.
 |
While the feasibility and potential utility of intraoperative optical coherence tomography has been demonstrated both in clinical studies and in real-world usage, several areas of enhancement are needed to enable iOCT to be part of a seamless surgical feedback platform. Image quality, improved efficiency, OCT-compatible surgical instrumentation, automated analysis software, instrument tracking systems and enhanced visualization systems are all areas of focus for improvement for the future. This update will focus on iOCT’s development, especially over the past five years, and potential future directions for technical advances.
The Latest Advances
Intraoperative OCT systems have advanced in several ways in recent years in an effort to make them more useful to surgeons.
• OCT-compatible surgical instruments.Current metallic surgical instruments limit visualization of the iOCT during real-time maneuvers, particularly in posterior segment surgeries. Light scattering and shadowing from metallic instruments limit visualization of the tissue-instrument interaction.1,2,3Studies have demonstrated that static imaging (i.e., stop and scan) is preferred in most posterior segment cases, which may be related to the lack of OCT-friendly instrumentation.4Semi-transparent materials with diffuse light-scattering profiles yield better compatibility with OCT, resulting in greater visibility of the instrument tip and less shadowing of the underlying tissue.3
 |
Surgical tools with OCT-compatible tips are currently under development. Ex vivo experience with these instruments has demonstrated excellent visualization of the instrument tip edges and angles in cadaver eyes. Shadowing was minimal to none with membrane scrapers and a surgical pick. With vitreoretinal forceps, a slight increase in shadowing was noted, due to the instrument’s more complex design.5
Further research into instrument reliability and subsequent advances in OCT-compatible surgical instruments are needed to maximize the usefulness of real-time iOCT. Beyond material changes, image processing solutions, such as spatial compounding, have been explored to minimize underlying shadowing while maximizing instrument visualization.2
• Instrument tracking systems. Precise instrument tracking may be essential for the seamless integration of real-time iOCT into the operating room, and to maximize its clinical utility. Acquisition of optimal OCT images likely requires a high sampling density and fast frame rate, which result in a limited field of view. In current systems, it’s challenging to maintain the iOCT centration at the point of interest while performing surgical maneuvers. Opportunities for tracking include using the microscopic video feed for identification of the instrument’s tip by machine-learning algorithms.6 Another option is to track the instrument handles using stereo cameras. The 3D position of the instrument tip is then computed based on the instrument geometry.7 Research is needed to evaluate these various approaches to tracking.
• Intraoperative Volumetric OCT. In current commercial iOCT systems, real-time OCT visualization is currently limited to B-scans of variable orientation. Volumetric visualization provides a unique opportunity for real-time iOCT. Current spectral domain systems are unable to create live volumetric imaging due to low scan speeds, computational challenges with volume rendering and the high requirements of postprocessing. The significantly increased imaging speed associated with new graphics-processing technologies and new swept-source OCTs has enabled real-time volumetric visualization.8
• Visualization of the OCT data stream. One of the most important advances that came with microscope-integrated iOCT systems was giving the surgeon the ability to have concurrent visualization of the OCT data stream and the surgical field by injecting the OCT data stream into the microscope ocular.3 More recently, advances in 3D display technologies and 4K high-definition visualization have enabled surgical procedures to be performed using these digital systems.4,9 The feasibility of using iOCT visualization in conjunction with 3D digital systems has also been demonstrated.10 In these systems, the OCT B-scans are displayed on the large-screen monitor simultaneously with the image of the surgical field. Emerging visualization technologies, such as immersive visors, provide additional opportunities for integration.
• Additional areas of development. Other areas of interest for emerging improvements are automated segmentation software, virtual reality visualization and robot assisted, iOCT-guided surgeries.6 Further research is needed, however, to improve these technologies to a level of reliability that’s sufficient for the OR.
Clinical Utility
Though the latest survey of members of the American Society of Retina Specialists (presented at the recent ASRS meeting in Chicago) found that only a quarter of the respondents have used iOCT, the potential benefits of iOCT have been demonstrated in various anterior and posterior segment procedures, including lamellar keratoplasty, cataract surgery, glaucoma procedures, membrane peeling procedures and retinal detachment repair.4,11,12-16
The number of studies investigating the clinical impact of iOCT is increasing each year. PIONEER was the first large scale (n=531) prospective clinical study that examined the potential benefits of iOCT with a microscope-mounted portable OCT setup. This study demonstrated that iOCT informed surgical decision-making in a significant number of anterior and posterior segment procedures.11 DISCOVER, the largest prospective study to date (n=837 at year three, now more than 2,000 subjects), demonstrated that microscope-integrated OCT provided intraoperative information that impacted surgical decision-making in 43 percent and 29 percent of anterior and posterior segment cases, respectively.4 Additional studies have demonstrated similar results regarding the impact and value of iOCT for a given surgical procedure, as well as providing evaluations of system ergonomics and iOCT functionality.4,9,10,14,16
• Anterior segment surgery. One of the most frequently reported applications for iOCT in the anterior segment is lamellar keratoplasty procedures, in particular Descemet’s stripping automated endothelial keratoplasty and Descemet’s membrane endothelial keratoplasty. Multiple studies have described the potentially important role for iOCT in evaluating graft apposition and subclinical interface fluid in DSAEK procedures.10, 14,15,18 Also, DMEK has been shown to potentially benefit from iOCT when it’s used to identify graft orientation and apposition and to limit the need for the S-stamp technique.19,22
In cataract surgery, iOCT may be helpful for evaluating corneal incision morphology, posterior capsule status and postoperative intraocular lens position. One study found that iOCT yielded anterior chamber depth measurements, posterior capsule assessments and IOL calculations that were more accurate than preoperative biometry measurements.16 Data related to iOCT utility in glaucoma procedures is more limited compared to other anterior segment procedures. In addition to aiding visualization of the ocular anatomy and confirming surgical goals, potential applications of iOCT in glaucoma surgery include determination of scleral graft depth, detecting any plugging of the iris and helping with proper tube placement.13
• Posterior segment surgery. In posterior segment surgeries, iOCT provides immediate feedback that can influence surgical maneuvers and management plans or confirm surgical endpoints. Studies have suggested that iOCT may add valuable information in more than half of cases and may alter surgical decision-making in nearly 30 percent of them.4
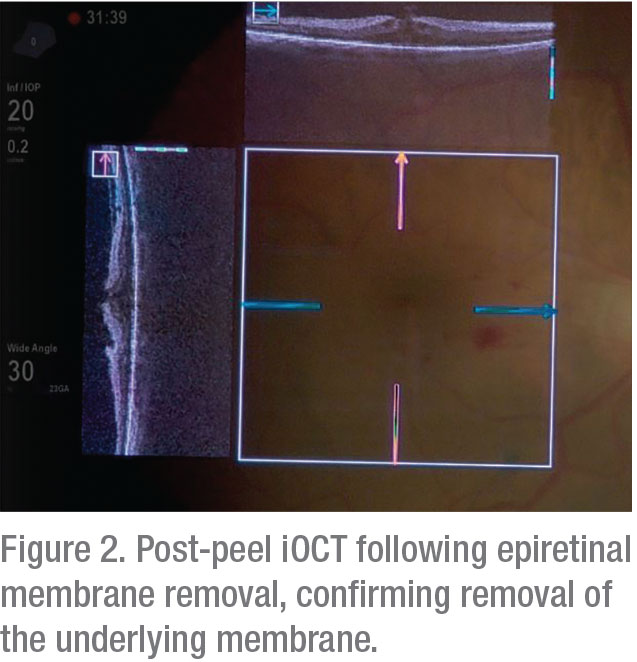 |
The potential clinical utility of iOCT appears to be particularly high in membrane-peeling cases.11,17,20 In these cases, iOCT can provide crucial information about the presence of residual membrane. DISCOVER showed disagreement between iOCT findings and surgeon impressions on the completeness of membrane peel in 26.8 percent of the patients. iOCT findings both prevented unnecessary maneuvers by confirming the surgical goal had been reached in some cases and identified the need for additional peeling to remove residual membranes in others.4
Beyond membrane peeling, studies have shown a potential role for iOCT in other procedures, including retinal detachments, proliferative diabetic retinopathy and vitreous hemorrhage.4,14,21 In addition, more complex procedures have been described using iOCT feedback and may benefit from intraoperative imaging, such as retinal prosthesis placement, retinal biopsy and delivery of targeted therapeutics (e.g., gene therapy, stem cell therapy and pharmacotherapy).4,23-25 In these cases, iOCT may provide information about tissue configurations, visualize the tissue/implant interface and confirm the location and volume of therapeutic delivery.4,23-25
In conclusion, integration of OCT into the operating room is a potential paradigm shift in surgical visualization during ophthalmic surgery. Rapid advances in technology in recent years have allowed for the introduction of commercial iOCT systems. Significant advances are still needed in order for iOCT to reach its full potential, however, and only a small percentage of surgeons have used it. Upcoming technologies may expand the usefulness of iOCT in a wider variety of conventional and emerging interventions. REVIEW
Dr. Sevgi is a research fellow at the Cleveland Clinic. Dr. Ehlers is The Norman C. and Donna L. Harbert Endowed Chair for Ophthalmic Research and director of The Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research at the Cleveland Clinic’s Cole Eye Institute.
Dr. Sevgi has no financial interests.
Dr. Ehlers’ disclosures are as follows:
Consultant to Leica, Zeiss, Alcon, Thrombogenics, Regeneron, Genentech, Allegro, Allergan, Roche and Novartis.
Research and Equipment Support: Alcon, Regeneron, Novartis, Genentech, Thrombogenics and Boehringer-Ingelheim.
1. Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci 2011;52:6:3153-9.
2. Ehlers JP, Tao YK, Farsiu S, et al. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina 2013;33:1:232-6.
3. Ehlers JP, Srivastava SK, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: Microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS One 2014;9:8:e105224.
4. Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER Study 3-Year Results: Feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery. Ophthalmology 2018;125:7:1014-27.
5. Ehlers JP, Uchida A, Srivastava SK. Intraoperative optical coherence tomography-compatible surgical instruments for real-time image-guided ophthalmic surgery. Br J Ophthalmol 2017;101:10:1306-08.
6. Carrasco-Zevallos OM, Viehland C, Keller B, et al. Review of intraoperative optical coherence tomography: Technology and applications [Invited]. Biomed Opt Express 2017;8:3:1607-37.
7. El-Haddad MT, Tao YK. Automated stereo vision instrument tracking for intraoperative OCT guided anterior segment ophthalmic surgical maneuvers. Biomed Opt Express 2015;6:8:3014-31.
8. Carrasco-Zevallos OM, Keller B, Viehland C, et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci Rep 2016;6:31689.
9. Kumar A, Hasan N, Kakkar P, et al. Comparison of clinical outcomes between “heads-up” 3D viewing system and conventional microscope in macular hole surgeries: A pilot study. Indian J Ophthalmol 2018;66:12:1816-19.
10. Ehlers JP, Uchida A, Srivastava SK. The integrative surgical theater: Combining intraoperative optical coherence tomography and 3d digital visualization for vitreoretinal surgery in the DISCOVER study. Retina 2018;38 Suppl 1:88-96.
11. Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) study: 2-year results. Am J Ophthalmol 2014;158:5:999-1007.
12. Khan M, Ehlers JP. Clinical utility of intraoperative optical coherence tomography. Curr Opin Ophthalmol 2016;27:3:201-9.
13. Kumar RS, Jariwala MU, V SA, et al. A pilot study on feasibility and effectiveness of intraoperative spectral-domain optical coherence tomography in glaucoma procedures. Transl Vis Sci Technol 2015;4:2:2.
14. Tao J, Wu H, Chen Y, et al. Use of iOCT in vitreoretinal surgery for dense vitreous hemorrhage in a Chinese population. Curr Eye Res 2019;44:2:219-24.
15. Titiyal JS, Kaur M, Falera R. Intraoperative optical coherence tomography in anterior segment surgeries. Indian J Ophthalmol 2017;65:2:116-21.
16. Fram NR, Masket S, Wang L. Comparison of intraoperative aberrometry, OCT-based IOL formula, Haigis-l, and Masket formulae for IOL power calculation after laser vision correction. Ophthalmology 2015;122:6:1096-101.
17. Pfau M, Michels S, Binder S, et al. Clinical experience with the first commercially available intraoperative optical coherence tomography system. Ophthalmic Surg Lasers Imaging Retina 2015;46:10:1001-8.
18. Juthani VV, Goshe JM, Srivastava SK, et al. Association between transient interface fluid on intraoperative OCT and textural interface opacity after DSAEK surgery in the PIONEER study. Cornea 2014;33:9:887-92.
19. Cost B, Goshe JM, Srivastava S, et al. Intraoperative optical coherence tomography-assisted Descemet membrane endothelial keratoplasty in the DISCOVER study. Am J Ophthalmol 2015;160:3:430-7.
20. Dayani PN, Maldonado R, Farsiu S, et al. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina 2009;29:10:1457-68.
21. Ehlers JP. Intraoperative optical coherence tomography: Past, present, and future. Eye (Lond) 2016;30:2:193-201.
22. Saad A, Guilbert E, Grise-Dulac A, et al. Intraoperative OCT-Assisted DMEK: 14 Consecutive Cases. Cornea 2015;34:7:802-7.
23. Gregori NZ, Lam BL, Davis JL. Intraoperative use of microscope-integrated optical coherence tomography for subretinal gene therapy delivery. Retina 2019. [published online first: 2017/04/21]
24. Ehlers JP, Petkovsek DS, Yuan A, et al. Intrasurgical assessment of subretinal tPA injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina 2015;46:3:327-32.
25. Ehlers JP, Ohr MP, Kaiser PK, et al. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina 2013;33:7:1428-34.



