The buzz about new treatments for age-related macular degeneration is intense. Several clinical trials examining treatments for choroidal neovascularization secondary to AMD will report results this year. Each treatment has a mechanism of action that deserves more attention, and this article will focus on these mechanisms in detail.
AMD and Its Progression
Ninety percent of AMD cases are the "dry" form, while the remainder are "wet" (exhibiting CNV).1
The etiology of AMD is poorly understood, but we know about its progression. Drusen, which comes from the German word druse, meaning gland, are deposits on Bruch's membrane that signify a risk of developing AMD. Small drusen (63 µm or smaller) are a risk factor for developing larger drusen. When drusen expand and develop indistinct borders, they're known as "soft," and increase the risk for AMD.
The risk of developing unilateral CNV in patients with drusen is 8 percent.2 In patients with CNV, the risk of developing it in the fellow eye is between 5 and 14 percent.3 In the clinic, fluorescein angiography defines the location and characteristics of CNV. Some cases of CNV are extrafoveal, making them easier to treat than subfoveal, because the overlying tissue in extrafoveal cases isn't as sensitive.
An angiogram also categorizes lesions into classic, occult or a combination. Classic lesions exhibit discrete areas of hyperfluorescence in the early phase of the angiogram with some pooling of the dye, but occult lesions aren't as well demarcated or bright. Classic lesions usually affect vision to a greater extent.
Current Treatments
Treatments for AMD include nutritional supplementation, laser photocoagulation and photodynamic therapy. Nutritional supplementation using antioxidants (vitamins C, E, and beta carotene) plus zinc has been shown to prevent progression of AMD in 25 percent of patients with moderate to advanced AMD.4 Supplements have encouraging data, but don't meet U.S. Food and Drug Administration standards to obtain an indication.
Photocoagulation can be effective in slowing vision loss, but has a high recurrence rate of CNV.5
PDT with verteporfin (Visudyne) is also effective in several causes of CNV in addition to AMD.6 Visudyne is administered intravenously, localizes in regions of CNV, and is activated with a low intensity non-thermal laser to close the abnormal blood vessels found in predominately classic subfoveal CNV. One study reports that 90 percent of patients needed retreatments after three months.7
 |
The new treatments closest to filing for approval work higher in the biochemical chain of events that causes angiogenesis than PDT with verteporfin. The new treatments in the later stages (phase III trials) of development are two anti-VEGF drugs, ranibizumab (Lucentis, Genetech) and pegaptanib sodium (Macugen, Eyetech); and a steroid derivative, anecortave acetate (Retaane, Alcon).
The New Targets
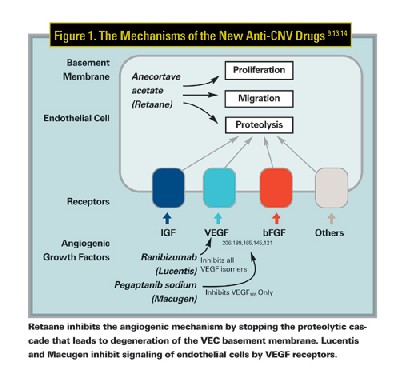
For the angiogenesis to occur, vascular endothelial cells (VECs) must be stimulated by angiogenic factors, including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). The angiopoietin molecules (Ang1 and Ang2) enhance the effects of VEGF. Upon activation by one or more of these factors, VECs secrete proteinases, which degrade proteins in the endothelial cell wall and allow proliferation and migration of new cells into the extracellular space (See Figure 1), the end result being new blood vessel creation.
VEGF Focuses on Signaling
It is important to understand why angiogenic stimulators like VEGF are stimulating this pathological process. Initially, the work of Judah Folkman, MD, of Children's Hospital in Boston, laid the foundation for research into distinct angiogenic factors that could be modulated pharmacologically. One factor that was found to have significant potential was vascular endothelial growth factor (VEGF), identified as an angiogenic mitogen in 1989.8
VEGF is produced by several different ocular cells, including glial cells, retinal pigment epithelium cells and even endothelial cells.9 The production of VEGF is usually a reaction to an ischemic environment due to disease. Levels of VEGF have been described as elevated in patients with CNV from a variety of causes, implicating it as one of the important mediators of angiogenesis. VEGF is required for normal vascular development and retinal vascularization.10,11
All three agents inhibit a portion of the stimulation/proliferation of VECs. Retaane functions by inhibiting the angiostatic proteolytic pathway. Macugen and Lucentis bind extracellular VEGF, preventing stimulation of VECs.
Anti-VEGF Therapy
Macugen is an aptamer, a polyethylene glycol oligonucleotide that binds one specific isomer of VEGF, VEGF 165, with high specificity in vivo. These molecules act like antibodies, binding VEGF. This inhibits signaling of endothelial cells by the VEGF receptors and prevents angiogenic stimulation of endothelial cells. The drug is delivered via an intravitreal injection. The therapeutic molecule has been modified with the addition of the polyethylene glycol attachment to lengthen the time between treatments by increasing the half-life of the drug. With this modification, re-injection every six weeks is required.12
Lucentis, the active fragment of a recombinant humanized antibody to VEGF, inhibits stimulation from all isoforms of human VEGF. Lucentis is delivered as an intravitreal injection, and patients must be retreated every four weeks. Phase III trials are currently enrolling patients and Lucentis could receive FDA approval in 2007.
Both drugs use intravitreal injection for delivery, which risks certain complications. The incidence of endophthalmitis with intravitreal delivery was reported as 1.3 percent, higher than the 0.03 percent recently reported for cataract extraction. (Miller JJ, ARVO Abstract #520, 2004) An extraocular mode of delivery for these drugs that maintains their effectiveness but lowers the risk of infection would be optimal. VEGF therapies have shown strong anti-angiogenic effects. We also don't know if other angiogenic factors, such as bFGF, stimulate endothelial cells even after VEGF is inhibited.
A New Class of Angiostatic Agents
Retaane is the first of a new class of drugs called angiostatic cortisenes. It was engineered from a steroid parent molecule, and retains a therapeutic benefit in treating CNV. Compared to placebo, Retaane has not yet shown any increases in intraocular pressure or cataract progression, side effects usually associated with old glucocorticoid steroids. Retaane inhibits angiogenesis by stopping the proteolytic cascade that leads to degeneration of the VEC basement membrane. By acting downstream from angiogenic signaling, Retaane inhibits angiogenesis regardless of which factor stimulated the VEC. VEGF, bFGF and Ang1 are all present in CNV lesions and therefore anecortave acetate is potentially effective at modulating CNV from any factor.
Retaane is delivered to the posterior extraocular surface by a novel, in-office procedure known as posterior juxtascleral depot (PJD) injection. The characteristics of Retaane allow therapeutic levels of the drug to reach the choroid and the retina. For PJD, a specially designed cannula is used to place the drug on the sclera close to the macula without penetrating the globe. In the currently ongoing trials, retreatment is required every six months. PJD is considered a relatively patient-friendly procedure due to the low risk, the long duration between treatments and the minimally invasive nature of the administration. Retaane is projected to receive FDA approval sometime in mid-2005.
With these and other exciting drugs on the horizon, the therapeutic implications for patients with vision loss due to CNV have never been more positive.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals.
Dr. Parver is a clinical professor of ophthalmology at Georgetown University.
Mr. Thomson is a research associate at Ophthalmic Research Associates in North Andover.
1. Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study Monograph. Surv Ophthalmol 1980;24(S):335-610.
2. Holtz FG, Wolfensberger TJ, Piguet B, et al. Bilateral Macular Drusen in Age-Related Macular Degeneration: Prognosis and Risk Factors. Ophthalmology 1994;101:1522-1528.
3. Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related Macular Degeneration: Etiology, Pathogenesis and Therapeutic Strategies. Survey of Ophthalmology 2003;48:3: 257-293.
4. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with Vitamins C and E, Beta Carotene and Zinc for Age-Related Macular Degeneration and Vision Loss. Archives of Ophthalmology 2001;119:1417-1436.
5. Moisseiev J, Alhalel A, Masuri R, Treister G. The impact of the Macular Photocoagulation Study results on the treatment of exudative age-related macular degeneration. Archives of Ophthalmology 1995;113:185-189.
6. Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial--VIP report no. 3. Ophthalmology 2003;110:4:667-73.
7. TAP Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. Archives of Ophthalmology 1999;117:1329-1345.
8. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:4935:1306.
9. Aiello LP. Vascular endothelial growth factor and the eye: Biochemical Mechanisms of action and implications for novel therapies. Ophthalmic Research 1997;29:354-362.
10. Carmeliet P, Ferreira V, Breir G. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380:435-439.
11. Aiello LP, Pierce EA, Foley ED. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluable VEGF receptor chimeric proteins. Proc Natl Acad Sci 1995;92:10,457-10,461.
12. Eyetech Study Group. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 2003;110:5:979-86.
13. Clark AF, Yorio T. Ophthalmic Drug Discovery. Nat Rev Drug Discov 2003;2:448-459.
14. Ferrara N, Houck HL, Jakeman LB, et al. The Vascular Endothelial Growth Factor Family of Polypeptides. Journal of Cellular Biochemistry 1991;47:211-218.