The discovery of human induced pluripotent stem (hiPS) cells has opened a new avenue for the treatment of degenerative diseases, like AMD, by using a patient’s own stem cells to generate tissues and cells for transplantation. For transplantation to be viable in AMD, researchers have to first figure out how to program the naïve hiPS cells to function and possess the characteristics of the native RPE cells that die off and lead to AMD. The research conducted by the Georgetown scientists shows that this critical step in regenerative medicine for AMD has greatly progressed. “This is the first time that hiPS-RPE cells have been produced with the characteristics and functioning of the RPE cells in the eye. That makes these cells promising candidates for retinal regeneration therapies in age-related macular degeneration,” says the study’s lead author Nady Golestaneh, PhD, assistant professor in GUMC’s Department of Biochemistry and Molecular & Cellular Biology. Using an established laboratory stem cell line, Dr. Golestaneh and her colleagues show that RPE generated from hiPS cells under defined conditions exhibit ion transport, membrane potential, polarized VEGF secretion and gene expression profile similar to those of a normal eye’s RPE. “This isn’t ready for prime time though,” said Dr. Golestaneh. “We also identified some issues that need to be worked out before these cells are ready for transplantation, but overall, this is a tremendous step forward in regenerative medicine.” She explains that the hiPS-derived RPE cells show rapid telomere shortening, DNA chromosomal damage and increased p21 expression that cause cell growth arrest. This might be due to the random integration of viruses in the genome of skin fibroblasts during the reprogramming of iPS cells. Therefore, generation of viral-free iPS cells and their differentiation into RPE will be a necessary step towards implementation of these cells in clinical application, Dr. Golestaneh says. “The next step in this research is to focus on a generation of ‘safe’ as well as viable hiPS-derived somatic cells,” she concludes.
| IOVS Publishes Consensus Findings on MGD |
|
The first global consensus report on meibomian gland dysfunction has been published in a special issue of Investigative Ophthalmology & Visual Science. The report is the result of findings from a two-year-long workshop composed of more than 50 leading clinical and basic research experts from around the world.
The workshop participants used an evidence-based approach to develop a worldwide definition: Meibomian gland dysfunction is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. This may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease. Using the same methodology, the participants developed a universal classification system—based on pathophysiology, rather than anatomical changes or the severity of disease—to meet the needs of clinicians and researchers alike. The consensus paper further proposes recommendations for diagnosing MGD and MGD-related disorders and presents a sequence of diagnostic tests to be performed in an order that will minimize the extent to which one test influences those that follow. Also included in the report are recommendations for the evaluation and grading of the severity of MGD, management of and therapy for the disease and norms for clinical trials designed to evaluate pharmaceutical interventions for treatment. The International Workshop was conducted by the Tear Film & Ocular Surface Society. While the breadth and depth of the consensus findings are expected to have a far-reaching impact on the clinical care of patients, the group of experts concur that additional research must be conducted to further study other aspects of MGD. These include its association with dry-eye disease and standardized and validated ways to identify symptoms and signs of MGD. |
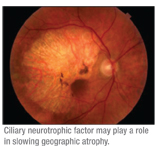
A Phase II clinical trial for the treatment of a severe form of geographic atrophy has become the first study to show the benefit of a therapy to slow the progression of vision loss for the disease. The results highlight the benefit of the use of a neurotrophic factor to treat GA and provide hope to nearly 1 million Americans suffering from GA.
The multicenter research team, including Kang Zhang, MD, PhD, of the University of California, San Diego, Shiley Eye Center, the lead author of the paper and one of the leading investigators in the study, found that long-term delivery of ciliary neurotrophic factor served to re-nourish the retina and stop or slow the loss of visual acuity caused by the disorder. The results were recently published online in the Proceedings of the National Academy of Sciences.
Dr. Zhang, a professor of ophthalmology and human genetics at the UCSD School of Medicine and director of UCSD’s Institute of Genomic Medicine, said there is currently no effective treatment for dry AMD or GA, though there is a very big need. “This could open the door to long-term treatment of dry AMD, using a simple surgical procedure,” he said.
In the trial, high-dose CNTF was delivered to 27 GA patients using encapsulated cell therapy (ECT). Another 24 patients received either a sham surgery (12) or a low-dose of CNTF (12). CNTF affects survival and differentiation of cells in the nervous system, including retinal cells. CNTF has been shown to retard the loss of photoreceptor cells in many animal models of retinal degeneration.
 |
There was a statistically significant difference in the change of the total macular volume in the eyes of study participants at the 12-month point, versus baseline in the high-dose group, according to Dr. Zhang. “In addition, all but one of the patients in the high-dose group, or 96.3 percent, maintained stabilized vision, compared to only 75 percent of the patients in the sham-treatment group,” he said.
The patients treated with a high dose of CNTF also showed an increase in retinal thickness as early as four months after implant, an increase that correlated to the stabilization of vision.
Development Error Causes Cataracts, Glaucoma
A research team in Boston has shown that RNA granules—key players in messenger RNA (mRNA) processing—can affect eye development, leading to juvenile cataracts in humans and mice. The research, published in the March 25 issue of Science, also demonstrates the first connection between RNA granules and glaucoma, as the humans and mice in the study developed glaucoma.
The work, in collaboration with researchers at Brigham and Women’s Hospital and Harvard Medical School, was conducted in the laboratory of Jackson Professor and Howard Hughes Medical Institute investigator Simon John, PhD. Dr. John and coauthors Stephen Kneeland, PhD, and Gareth Howell, PhD, identified a malfunctioning gene in a mouse strain that develops both cataracts and glaucoma. The gene, TDRD7, fails to build an essential protein and disrupts the development of the mouse eye lens. Mice missing the protein developed high intraocular pressure and optic nerve damage as well as cataracts.
The research teams combined forces with Salil A. Lachke, PhD, of Brigham and Women’s Hospital and Harvard Medical School, who had earlier found the same malfunction in a study of genetic data from patients with pediatric cataracts. They discovered that the protein missing in the children and the mice belongs to a type of structure known as RNA granules. RNA granules function to regulate mRNAs in the cell. mRNA’s primary job is to serve as a template to carry DNA-encoded information from the nucleus into the cytoplasm or body of the cell, providing the blueprints for protein production. The TDRD7 mutation affects mRNA regulation, and this misregulation was implicated in causing the cataracts. Further, the human patients developed glaucoma following cataract extraction.
TDRD7 deficiency greatly reduces the number of stress granules that are produced in lens cells in response to oxidative stress, the researchers show. Stress granules, a specific type of RNA granule, are important to protect the cell in stressful conditions. Oxidative stress has been previously suggested to contribute to glaucoma by damaging the ocular drainage structures. The new findings imply that mice and patients with these mutations may not have adequate protection from oxidative stress in the drainage structures of the eye. With increasing age, their tissues may be more susceptible to oxidative damage, resulting in high intraocular pressure and glaucoma.
Although further experiments are needed to be certain, this work is the first to suggest that RNA granules are important in modulating the oxidative stress response relevant to glaucoma. Dr. John notes, “There is a growing body of literature indicating that if you disturb oxygen levels in the eye—including after cataract surgery—the risk of developing glaucoma increases.”
Dr. John says that mutations in the TDRD7 gene could cause a double jeopardy for childhood glaucoma. “First, they cause cataract, and cataract extraction may raise oxidative stress in the ocular drainage tissues,” he says. “Second, they impair the formation of protective stress granules in response to oxidative stress.”
Jackson research scientist Richard Smith, MD, a former ophthalmologist now in the John lab, notes that while pediatric cataracts are relatively rare in the United States (occurring in about one in every 30,000 births), they are a major problem in other parts of the world, notably the Middle East. “In Saudi Arabia about one in 2,500 babies is born with juvenile cataracts,” Dr. Smith says.
First Look at Asian Americans’ Glaucoma Risk
It’s generally known that African Americans have the highest risk for glaucoma (about 12 percent) among racial groups in the United States. They are more than twice as likely as non-Hispanic white Americans (5.6 percent) to develop the disease. But little was known about risks for Asian Americans until a National Eye Institute-funded study published recently in Ophthalmology (online). By reviewing insurance records of more than 44,000 Asian Americans older than 40, the researchers found their glaucoma risk to be 6.5 percent, which is about the same as U.S. Latinos.
Racial-ethnicity risk rates help people and doctors plan for eye care and take extra precautions if appropriate. Since Asian Americans are the second fastest growing population in the United States—a trend likely to continue for years to come—such risk information is urgently needed.
The study also detailed the Asian American ethnic groups most likely to develop the three main types of glaucoma: open-angle; narrow-angle; and normal-tension.
The rate of NAG was higher in Asian Americans than in any other racial group in the study and highest of all among Chinese and Vietnamese Americans. The risk of NTG was three to 10 times higher in Japanese Americans than other Asian ethnicities studied, and nearly all of the Asian sub-groups were at higher risk than non-Asian Americans. Among Asian Americans, OAG rates were highest among Japanese Americans (about 9.5 percent), followed by Indian and Pakistani Americans (about 7.7 percent).
The study was led by Joshua D. Stein, MD, of the Kellogg Eye Center at the University of Michigan, who said the results have implications for patient care.
“For example, the inner eye angle anatomy of patients of Chinese or Vietnamese ancestry should be carefully examined,” Dr. Stein said. “And since NTG won’t be detected by simply measuring intraocular pressure, eye doctors need to assess the status of the optic nerve in patients whose ethnicity makes them more susceptible to this type of glaucoma,” he added. He and his coauthors recommend that future studies explore potential genetic and environmental reasons for some of the observed differences in glaucoma rates among the different races and Asian ethnicities.



