Presentation
A 71-year-old female presented to her local ophthalmologist with six months of painless gradual worsening vision in the right eye with associated redness. On examination, she was found to have no light perception and iris neovascularization in the right eye. On B-scan ultrasonography, a retinal detachment with possible intraocular blood or mass was discovered. The patient was then referred for evaluation by the Wills Eye Hospital Ocular Oncology Service.
History
The ocular history was only notable for a prior posterior vitreous detachment in the right eye. Medical history revealed multiple vascular risk factors, including a stroke two years prior that left her wheelchair-bound, type 2 diabetes mellitus of many years, hypertension and obesity. There was no family history of cancer. Social history was unremarkable, notably with no history of tobacco use. Current medications included aspirin, atenolol, prednisone and famotidine.
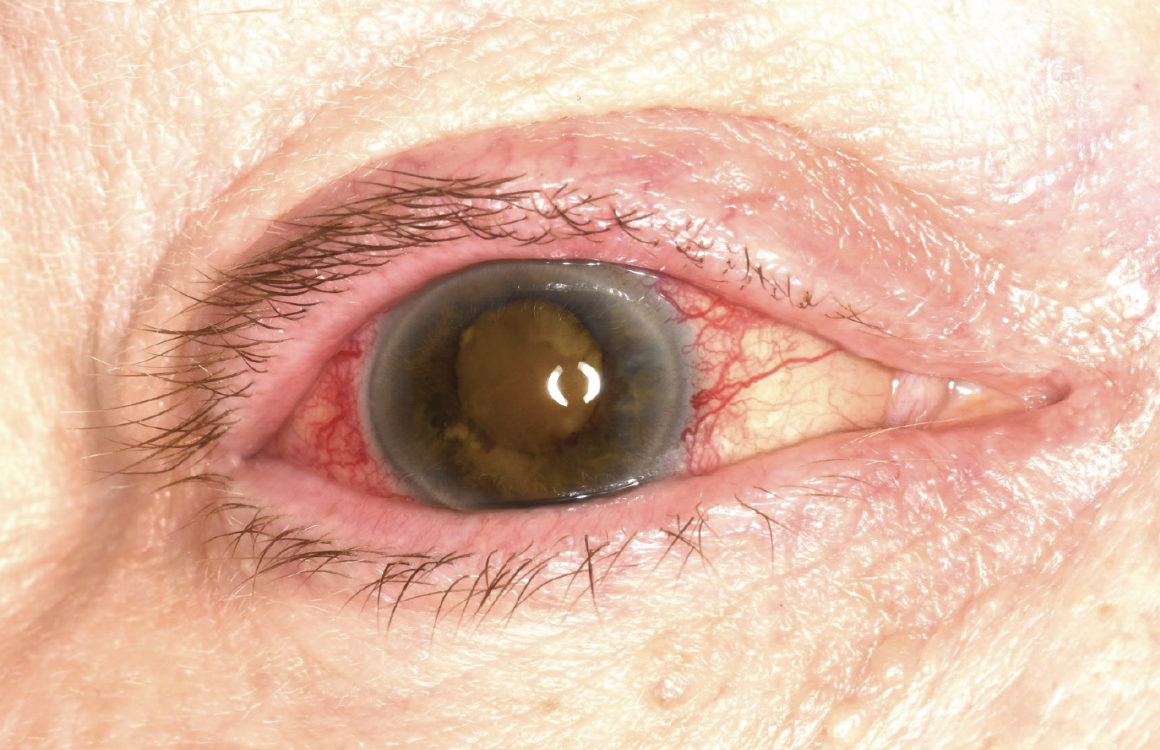 |
| Figure 1. External photograph of the right eye showing eyelid congestion and erythema, conjunctival/episcleral vascular injection, and dense cataract. Iris neovascularization with posterior synechiae was present, limiting dilation. |
Examination
On ocular examination, best-corrected visual acuity was no light perception in the right eye and 20/40 in the left eye. The right pupil was non-reactive and the left pupil was round and reactive without afferent pupillary defect. Intraocular pressure was 9 mmHg in the right and 15 mmHg in the left eye. Confrontation visual fields weren’t possible in the right eye and normal in the left eye. Extraocular movements were full in both eyes.
In the right eye, there were several additional findings including mild upper and lower eyelid congestion and erythema, marked conjunctival/episcleral vascular injection especially prominent at the limbus, neovascularization of the iris (NVI) with iris atrophy, peripheral anterior synechiae, posterior synechiae and a dense nuclear sclerotic cataract (Figure 1). Fundus examination and optical coherence tomography weren’t possible due to the dense cataract.
In the left eye, the anterior segment examination revealed moderate nuclear sclerotic cataract. Fundus examination and optical coherence tomography revealed a flat retina with minimal epiretinal membrane, macular edema and dot-blot hemorrhages.
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
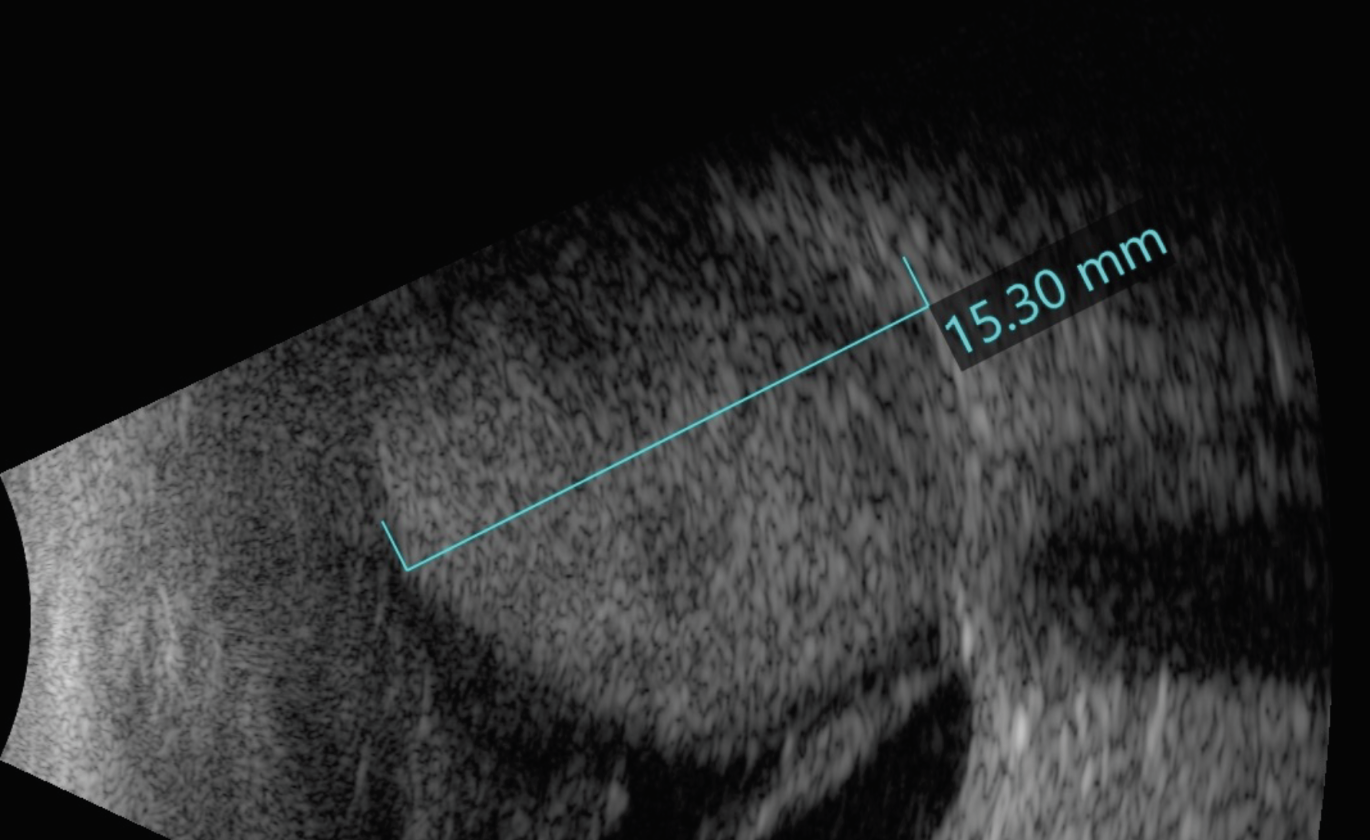 |
| Figure 2. B-scan ultrasonography of the right eye showing an elevated choroidal mass with central cavitation and with retinal detachment inferiorly. |
Work-up, Diagnosis and Treatment
On B-scan ultrasonography of the right eye, there was an elevated, echodense mass measuring 15 mm in thickness and 20 mm in diameter with a few lucencies, suspicious for tissue alteration, necrosis or cavitation. The mass demonstrated spontaneous vascular pulsations, suggestive of solid tumor rather than hemorrhage. There was adjacent retinal detachment (Figure 2). Magnetic resonance imaging revealed an intraocular mass that was hyperintense on T1-weighted images and hypointense on T2-weighted images (Figure 3). Importantly, gadolinium contrast showed enhancement within the mass, suggestive of solid tumor rather than hemorrhage or effusion. In the setting of a blind eye with intraocular mass concerning for choroidal melanoma, enucleation was recommended.
Following enucleation, pathology of the eye (Figure 4) grossly revealed a large intensely pigmented tumor arising from the ciliary body and choroid, as well as a 5 x 3 mm area of extraocular extension posteriorly. There were related sequelae from the tumor including extensive posterior synechiae with nearly the entire posterior surface of the iris adherent to the anterior surface to the lens, retinal detachment and dense cataract. Microscopic examination revealed tumor cells consistent with ciliochoroidal melanoma and tumor necrosis.
The patient recovered well from the enucleation and got fitted for a prosthesis. She was referred to a medical oncologist for systemic workup for metastatic disease. At this early point, there were no systemic metastases. Routine follow-up care was recommended.
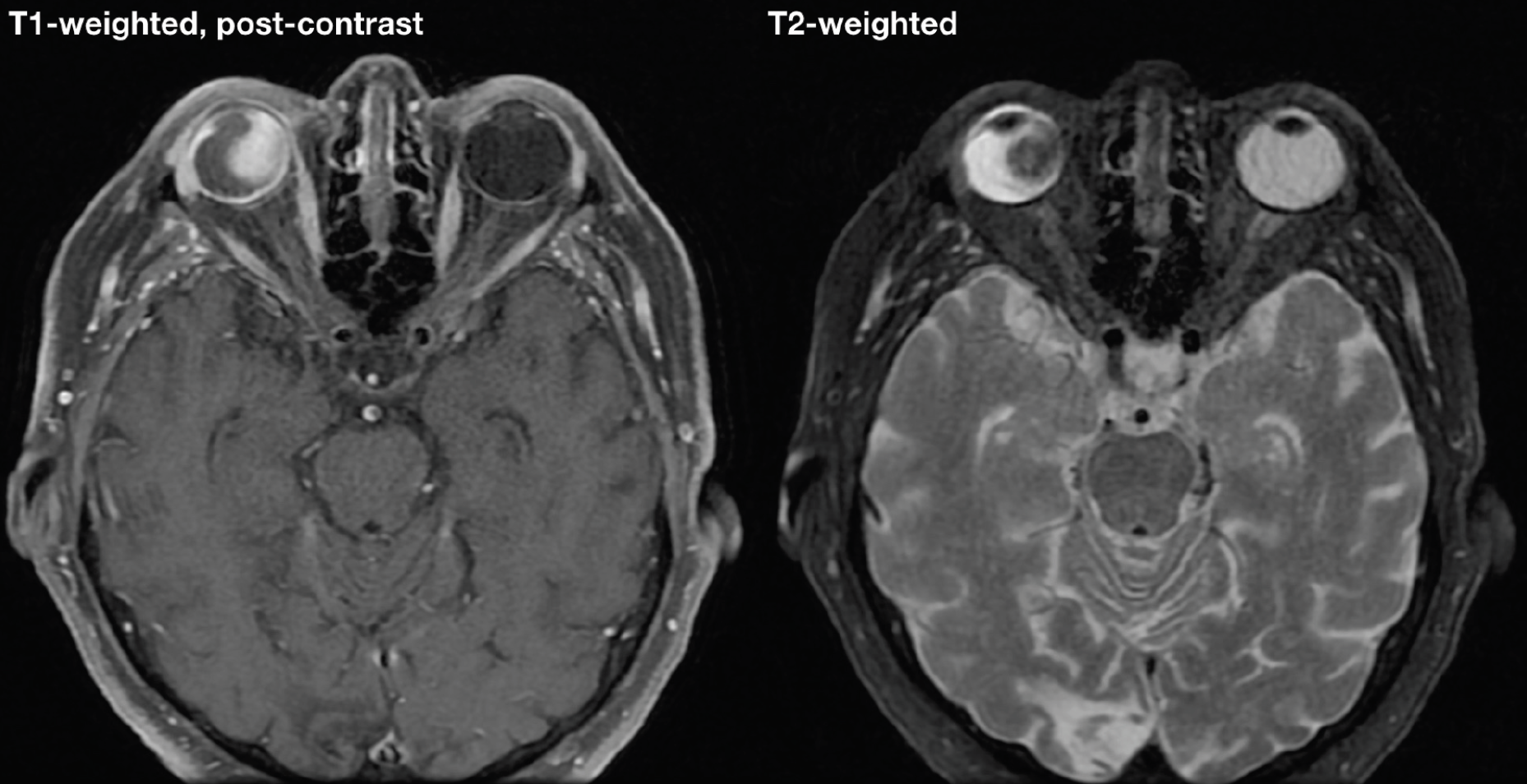 |
| Figure 3. Magnetic resonance imaging showing hyperintense enhancing mass on T1-weighted, fat-suppressed image and hypointense mass on T2-weighted image within the right eye. |
Discussion
This case of uveal melanoma in a patient presenting with NVI is unique for two reasons: first, in adults, intraocular tumors are a rare cause of NVI or neovascular glaucoma (NVI leading to secondary elevation of IOP). Second, NVI and NVG are seen relatively rarely in uveal melanoma.
The most common causes of NVI and neovascular glaucoma in adults are diabetic retinopathy, central retinal vein occlusion and ocular ischemic syndrome. In total, these top three causes account for 80 percent of all cases of NVI/NVG.1 A host of other causes make up the remaining 20 percent, including central retinal artery occlusion, uveitis, vasculitis, longstanding retinal detachment and neoplasms which include uveal melanoma, uveal metastasis and retinoblastoma.1 It’s important to note, however, that in children, the differential includes diverse ocular, genetic and systemic diseases, including intraocular tumors; thus, a full ophthalmological examination is needed to establish the underlying diagnosis.2
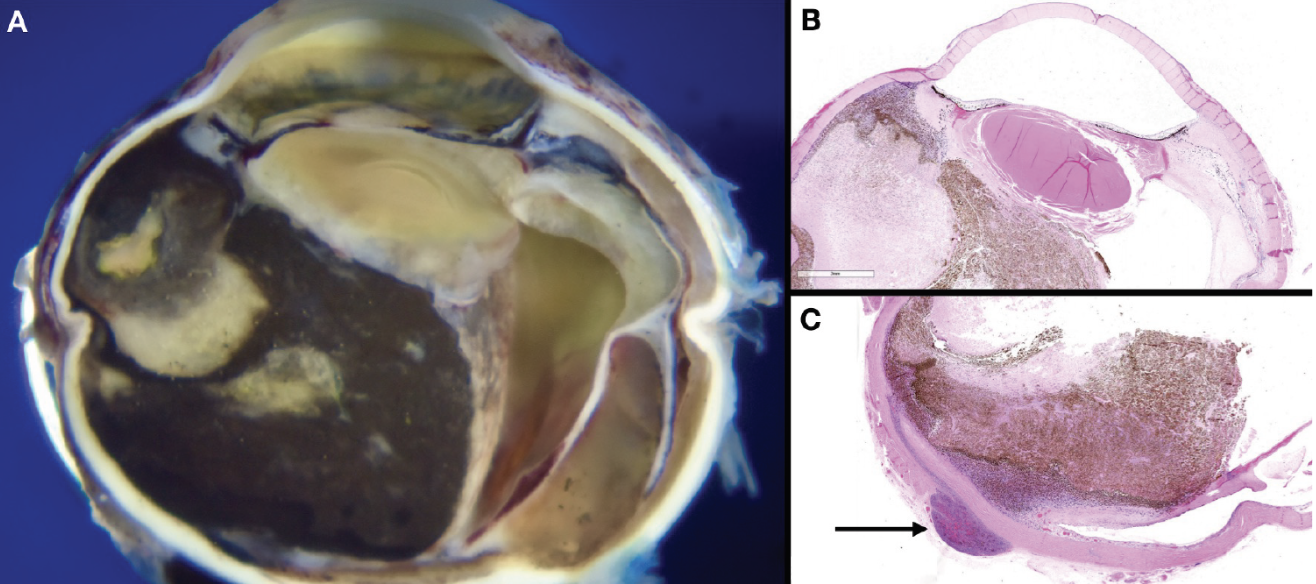 |
| Figure 4. Pathology photos of sections of enucleated right globe. Gross photograph (A); hematoxylin and eosin stained slides (B) and (C). The arrow indicates area of posterior extraocular extension. |
Uveal melanoma is rarely associated with secondary glaucoma. In a comprehensive review from the Wills Eye Hospital Ocular Oncology Service, only 3 percent of eyes with an intraocular tumor demonstrated secondary increase in IOP.3 The frequency and mechanism of IOP elevation varied based on the type of melanoma. Seven percent of iris melanomas demonstrated secondary IOP elevation, most commonly due to direct invasion of the angle; 17 percent of ciliary body melanomas demonstrated secondary IOP elevation, most commonly due to pigment dispersion or direct invasion of the angle; and only 2 percent of choroidal melanomas demonstrated secondary IOP elevation, most commonly due to NVI.3
It’s also valuable to discuss several clinical features of this case that are relevant for prognosis, namely tumor size, extraocular extension size, metastasis and genetic features.
Tumor size is measured by thickness and basal diameter. Small tumors (<3 mm thick and <11 mm basal diameter) have a 16 percent five-year mortality following enucleation; medium tumors, 35 percent mortality; and large tumors (>8mm thick or >15mm basal diameter), 53 percent mortality.4,5 Our patient’s tumor would be classified as large based on both thickness and basal diameter.
Extraocular extension is classified as microscopic with a 37 percent five-year mortality, small (1 to 4 mm) with 24 percent, and large (>5 mm) with 78 percent.4 Our patient’s tumor had a small area of extraocular extension.
Patients with choroidal melanoma demonstrate a high rate of metastasis: 32 percent by five years and 56 percent by 25 years, most commonly to the liver, lung and bone.6 Metastasis is associated with a high mortality despite treatment, with a median survival of approximately 10 months.7 As such, imaging surveillance for metastasis is recommended, with either MRI, computed tomography or hepatic ultrasonography at three to 12 month intervals depending on tumor features and cancer center-specific protocols.8 Given the poor prognosis in metastatic disease, there’s great interest in novel therapeutics for these patients. Several new targeted drugs are currently being investigated, such as tebentafusp, a bispecific protein that directly binds T cells to melanoma cells in order to activate the immune response,9 and darovasertib, a protein kinase C inhibitor that prevents melanoma cells from proceeding with the cell cycle.10 Metastatic uveal melanoma is the final frontier of treatment and continues to be an active area of research.
Genetic features have become an extremely important prognostic factor. The Cancer Genome Atlas is an international collaboration that’s created classification systems for many cancers based on molecular changes in cancer cells.11 For uveal melanoma, a TCGA group is assigned based on findings in chromosome 3 and 8, with more changes indicating higher risk.12 This patient ultimately declined genetic testing. However, it should be noted that genetic testing is widely used in ocular oncology as a routine part of counseling patients with uveal melanoma.
In conclusion, in an adult patient presenting with iris neovascularization, uveal melanoma is a rare but serious consideration that should remain on the differential diagnosis. Factors such as tumor size, extraocular extension, and genetic features help to predict prognosis and drive the treatment plan with the goal of reducing the risk of melanoma-related metastasis.
1. Dumbrăveanu L, Cușnir V, Bobescu D. A review of neovascular glaucoma: Etiopathogenesis and treatment. Rom J Ophthalmol 2021;65:4:315-329.
2. Nieves-Moreno M, Peralta J, Noval S. Neovascular glaucoma in children: A case series and a review of the literature. Eur J Ophthalmol 2022;32:6:3289-3294.
3. Shields CL, Shields JA, Shields MB, Augsburger JJ. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology 1987;94:7:839-46.
4. Kaliki S, Shields CL, Shields JA. Uveal melanoma: Estimating prognosis. Indian J Ophthalmol 2015;63:2:93-102.
5. Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol 1992;110:2:245-50.
6. Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye (Lond) 2017;31:2:241-257.
7. Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 2003;44:11:4651-9.
8. Rantala ES, Hernberg MM, Piperno-Neumann S, Grossniklaus HE, Kivelä TT. Metastatic uveal melanoma: The final frontier. Prog Retin Eye Res 2022;90:101041.
9. Nathan P, Hassel JC, Rutkowski P, IMCgp100-202 Investigators, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:13:1196-1206.
10. Cao L, Chen S, Sun R, Ashby CR Jr, Wei L, Huang Z, Chen ZS. Darovasertib, a novel treatment for metastatic uveal melanoma. Front Pharmacol 2023;14:1232787.
11. Wang Z, Jensen MA, Zenklusen JC. A practical guide to the cancer genome atlas (TCGA). Methods Mol Biol 2016;1418:111-41.
12. Jager MJ, Brouwer NJ, Esmaeli B. The cancer genome atlas project: An integrated molecular view of uveal melanoma. Ophthalmology 2018;125:8:1139-1142.



