For as long as we've had antibiotics, we've been fighting bacterial resistance. The first instances of resistance were observed shortly after the introduction of penicillin to the drug market in 1943, and it continues to be a rampant, incessant problem.1 Since 1881, when obstetrician Carl Crede published his ground-breaking work on neonatal gonococcal conjunctivitis,2 medical researchers have experimented with thousands of chemicals to treat and prevent ocular infections while minimizing resistance. Through this work, we've found that, though antibiotic resistance is inevitable, it can be slowed by appropriate prescription regimens and avoidance of indiscriminate usage. In this article, we'll explain how you can impede bacterial resistance in your practice.
The Mounting Resistance
Ocular infections such as bacterial conjunctivitis, keratitis and the less common endophthalmitis are caused by a variety of pathogens. The bacteria most frequently associated with conjunctivitis are Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae and Chlamydia trachomatis.3-5 Severe purulent conjunctivitis is caused by the bacterium Neisseria gonorrhoeae.6 Bacterial keratitis is normally caused by S. aureus, S. pneumoniae, or Staphylococcus epidermidis.7,8 The most common isolates from endophthalmitis conditions (as discussed in last month's "Therapeutic Topics") are generally species of Streptococcus and Staphylococcus.9 Many ocular pathogens have developed resistance to a variety of available antibiotics, including the newest additions, the fluoroquinolones.
The first bacterial pathogen to exhibit resistance to penicillin was S. aureus, which currently resists penicillin in 80 percent of strains.10 In the United States, the most common source of all bacterial infections is staphylococci, including methicillin-resistant S. aureus, which is of major concern due to its development of multiple-drug resistance.11
In the late 1940s and early 1950s, streptomycin, chloramphenicol and tetracycline were introduced, which launched the era of antibiotic chemotherapy. These antibiotics were effective against an assortment of gram-positive and gram-negative pathogens. However, by 1953, a strain of bacillus isolated in Japan was found to be resistant to chloramphenicol, tetracycline, streptomycin and the synthetically produced sulfanilamides. Various reports of the ability of bacteria to pass resistance genes between strains and even between species were rapidly escalating.10
In 1963, the first antibacterial quinolone, nalidixic acid, was approved for the treatment of urinary tract infections caused by gram-negative bacteria. Fluoroquinolones were made available in the early 1990s through the addition of fluorine to the quinolone structure. Fluoroquinolones are indicated for the treatment of ocular infections and display improved activity against gram-positive bacteria compared to the original quinolones. They have broad-spectrum coverage, rapid onset of action and the best ocular penetration of any topical antibiotic.12 Nevertheless, reports of ocular bacterial resistance to the second-generation fluoroquinolones, ciprofloxacin and ofloxacin, have compromised their success.13 A five-year review study reported that S. aureus resistance to both ciprofloxacin and ofloxacin dramatically increased from 5 percent in 1993 to 35 percent in 1997.8 Consequently, third- (levofloxacin) and fourth- (gatifloxacin and moxifloxacin) generation fluoroquinolones have recently been introduced to thwart this resistance.
Gatifloxacin (Zymar, Allergan) and moxifloxacin (Vigamox, Alcon), both approved in 2003, are active against fluoroquinolone-resistant Staphylococci and Streptococci as well as penicillin- and macrolide-resistant isolates.14 The addition of a methoxy side-chain at the R8 position and other structural modifications have allowed these new fluoroquinolones to maintain wide-spectrum coverage of gram-negative, gram-positive and anaerobic bacteria.
They've proven to be the most effective monotherapeutic agents for ocular bacterial infections, including bacterial keratitis and conjunctivitis.15 (Long M, et al. IOVS 2003;44:ARVO E-Abstract 2115. Dvorak AW, et al. IOVS 2004; 45:ARVO E-Abstract 4972) Despite obvious advantages of fourth-generation fluoroquinolones, however, researchers have identified double mutation isolates of S. aureus that are resistant to moxifloxacin and gatifloxacin in vitro.16,17
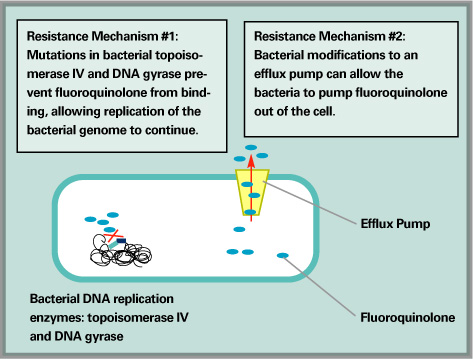
Over time, bacteria have developed two ways to defeat antibiotics.
Mechanisms of Resistance
Antibiotic resistance occurs when bacteria acquire genes that allow them to interfere with the antibiotic's mechanism of action either through spontaneous DNA mutation, transformation or transfer of plasmids. The b-lactam antibiotics inhibit penicillin-binding proteins (PBPs) in the bacterial cell wall. Bacteria such as staphylococci have developed resistance to b-lactam antibiotics by acquiring the ability to produce b-lactamase, which destroys the antibiotic, and by acquiring a novel PBP that is not susceptible to antibiotic inhibition.11 Streptococcus species actually alter their ribosomes to prevent the binding of erythromycin, tetracycline, streptomycin and gentamicin.18
Fluoroquinolones act by inhibiting DNA gyrase and topoisomerase IV, both of which are enzymes involved in bacterial DNA synthesis. However, fluoroquinolone resistance can develop either through alterations in the target enzymes or alterations in access to the target enzymes. Alterations in target enzymes include mutations that develop in DNA gyrase, which tend to occur in fluoroquinolone-resistant gram-negative bacteria or mutations in topoisomerase IV, which occur more commonly in fluoroquinolone-resistant gram-positive bacteria.19 Alterations in access to target enzymes prevent fluoroquinolones from crossing the bacterial cell wall. This mechanism of bacterial resistance involves the expression of multi-drug resistant membrane-associated efflux pumps, which actively pump the antibiotic out of the bacterial cell.20
Slowing Resistance
Ocular infections can be a source of concern or discomfort to patients, and may lead to vision loss in cases of bacterial keratitis and endophthalmitis without timely therapy. However, with the growing threat of bacterial resistance, it's necessary to use caution when prescribing antibiotics, in order to avoid indiscriminate usage. For example, in practice, over 80 percent of conjunctivitis cases are treated with antibiotics, though one study found that only 32 percent of all conjunctivitis cases tested actually had a bacterial origin.3 In general, studies indicate a culture positive rate of approximately 40 percent for clinically identified cases of bacterial conjunctivitis. Though errors in culture technique may partially account for this rate, it's accurate to conclude that in many conjunctivitis cases of non-bacterial origin, physicians are prescribing antibiotics.
• Antibiotics in practice. Ocular infections are caused by a variety of bacterial pathogens, and it would be a huge advantage to always obtain a culture before prescribing an antibiotic. However, this is far too expensive in terms of both economics and time. Thus, clinicians normally rely on medical history and symptomatology in determine proper diagnosis and subsequent treatment.
Initial treatment with fluoroquinolones has become a popular technique for the management of ocular infection. These agents, especially the fourth-generation 8-methoxyfluoroquinolones, provide broad-spectrum coverage, low toxicity, good ocular penetration, extended tear film concentration and easy availability.8
The fourth-generation fluoroquinolones are also attractive options because they are currently the agents closest to having the ideal characteristic of a low minimum inhibitory concentration at a tissue concentration high enough to prevent the development of mutations. In a recent study, the mean conjunctival concentration of moxifloxacin was found to be almost seven times higher than that of ciprofloxacin.21 Antibiotics can reach higher tissue concentrations in a variety of ways: through frequent dosage, increased drug concentration within the ophthalmic formulation, adjunctive delivery devices or enhanced drug penetration.
A low MIC can also be achieved by using a fluoroquinolone with heightened activity against the bacterial species causing the infection.19 For instance, a new ophthalmic solution of levofloxacin (Iquix, Vistakon) is under development, with an increased concentration of 1.5%, higher than the currently available 0.5%, for the treatment of bacterial corneal ulcers. If approved, this new formulation may provide enhanced protection against resistance.
• Fourth-generation first. Initial use of fourth-generation fluoroquinolones, forgoing the use of earlier-generation agents, may help avoid selection of resistant mutants due to the new drugs' dual mechanism of action, coupled with their enhanced potency and ability to reduce efflux from the bacterial cell. These improvements heighten their spectrum of activity to include strains of Streptococcus and Staphylococcus species that are otherwise resistant to the older fluoroquinolones.20
Bacterial resistance to a second-generation fluoroquinolone can occur with a single mutation, which means that one bacterium in 10 million can develop resistance. However, multiple mutations are required for fourth-generation fluoroquinolone resistance to occur, which is a likelihood of one in 10 trillion.22 Prolonged use of earlier-generation fluoroquinolones, however, can select for bacterial strains already possessing a mutation, which increases the potential for a second mutation, thus potentially conferring resistance to fourth-generation fluoroquinolones. This problem can be avoided with initial use of fourth-generation fluoroquinolones.
• Patient compliance. As much as the practitioner can do, there are some aspects that ultimately depend on patient compliance. Dose frequency can have a meaningful impact on compliance. A review of 76 publications that used electronic devices to track patient compliance with antibiotic dosing regimens demonstrated that compliance decreased with increased dose frequency, going from 79 percent (± 14 percent) for q.d. dosing to 51 percent (± 20 percent) for q.i.d. regimens.23
Compliance also depends on the length of treatment. In a study evaluating compliance by penicillin tablet count for streptococcal infections, researchers found that 44 percent of children were fully compliant on day three, but by day nine, compliance had fallen to 18 percent.24
Though compliance depends on many outside factors, doctors may influence some of them: Fully inform patients of the possible adverse events and other consequences associated with discontinuing their treatment. Directions for the prescribed dosing schedule should be simple, straightforward and always presented to the patient in writing.
So, what will it take to curb the development of bacterial resistance? How can we prolong the efficacy of the newest generation of antibiotics? It may seem as though our strongest ammunition, the fourth-generation fluoroquinolones, should be held in reserve in order to maintain powerful reinforcements for use only in situations that truly require them. However, these potent antibiotics may actually be our best bet for controlling resistance on the front lines. In general, clearing infections as quickly as possible is crucial in order to prevent the potential for bacteria to develop drug-resistant mutations. Of the available options, the fourth-generation fluoroquinolones, though not invincible, have proven to fit this profile the closest.
Though a uniform decision on behalf of the medical community regarding how to handle antibiotic therapy would be to our advantage, many questions remain unanswered. We do know that the best ways to combat bacterial resistance include the initial use of fluoroquinolones, accurate diagnosis of bacterial infections and improved patient compliance. Even using these tactics, the war on bacterial resistance may never be won, but we can still put up a good fight.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Ms. Plumer is a research associate at Ophthalmic Research Associates in North Andover.
1. Abraham E. Selective reminiscences of beta-lactam antibiotics: early research on penicillin and cephalosporins. Bioassays 1990;12:12:601-6.
2. Crede CSR. Die Verhutuna der augenentzundung der neugeborenen. Arch Gynakol 1881;18:367.
3. Rietveld RP et al. Predicting bacterial cause in infectious conjunctivitis: cohort study on informativeness of combinations of signs and symptoms. BMJ 2004;24;329:7459:206-10.
4. Block SL et al. Increasing bacterial resistance in pediatric acute conjunctivitis (1997-1998). Antimicrob Agents and Chemother 2000;44:6:1650-1654.
5. Malathi J et al. A hospital based study on the prevalence of conjunctivitis due to chlamydia trachomatis. Indian J Med Res 2003;117:71-5.
6. Mak DB et al. A large outbreak of conjunctivitis caused by a single genotype of neisseria gonorrhoeae distinct from those causing genital tract infections. Epidemiol Infect 2001;126:3:373-8.
7. Schaefer F et al. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol 2001;85:842-847.
8. Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis, a 5-year review. Ophthal 1999;106:7:1313-1318.
9. Jackson TL et al. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol 2003;48:4:403-23.
10. Todar K. Bacterial resistance to antibiotics. Todar's Online Textbook of Bacteriology 2002. http://textbookofbacteriology.net/resantimicrobial.html
11. Golemi-Kotra D et al. Resistance to beta-lactam antibiotics and its mediation by the sensor domain of the transmembrane B1aR signaling pathway in staphylococcus aureus. J Biol Chem 2003;278:20:18419-25.
12. Mah FS. Fourth-generation fluoroquinolones: new topical agents in the war on ocular bacterial infections. Curr Opin Ophthalmol 2004;15:316-320.
13. Chaudhry NA et al. Emerging ciprofloxacin-resistant pseudomonas aeruginosa. Am J Ophthalmol 1999;128:509-510.
14. Wise R. Maximizing efficacy and reducing the emergence of resistance. J Antimicrob Chemother 2003; 51:S1:37-52.
15. Hyon et al. 2004. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarythromycin in the treatment of experimental mycobacterium chelonae keratitis. Arch Ophthalmol 122:8:1166-1169.
16. Ince D, Zhang X, Hooper DC. Activity of and resistance to moxifloxacin in staphylococcus aureus. Antimicrob Agents and Chemother 2003;47:4:1410-1415.
17. Ince D, Hooper DC. Mechanisms and frequency of resistance to gatifloxacin in comparison to AM-1121 and ciprofloxacin in staphylococcus aureus. Antimicrob Agents and Chemother 2001;45:10:2755-2764.
18. Lewis R. The rise of antibiotic-resistant infections. FDA Consumer Mag 1995 http://www.fda.gov/fdac/features/795_antibio.html.
19. Hwang DG. Fluoroquinolone resistance in ophthalmology and the potential role for newer ophthalmic fluoroquinolones. Surv Ophthalmol 2004;49:S2:79-83.
20. Blondeau J. Fluoroquinolones: mechanisms of action, classification and development of resistance. Surv Ophthalmol 2004;49:S2:73-78.
21. Wagner RS et al. Evaluation of moxifloxacin and ciprofloxacin concentrations in human conjunctival tissue following administration of moxifloxacin 0.5% (Vigamox) and ciprofloxacin 0.3% (Ciloxan) ophthalmic solution. Arch Ophthalmol [in press].
22. Mather R, Karenchak, LM, Romanowski EG, et al. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol 2002;133:463-466.
23. Claxon AJ, Cramer J, Pierce C. A systematic review of the association between dose regimens and medication compliance. Clin Ther 2001;23:1296-310.
24. Bergman AB, Werner RJ. Failure of children to receive penicillin by mouth. New England J Med 1963;268:1334-8.