Though central serous chorioretinopathy is common, it can still be challenging to diagnose and treat, since it can present with a wide range of symptoms, such as blurred vision, a central scotoma and metamorphopsia (visual distortion); as well as with symptoms that are similar to other conditions, such as age-related macular degeneration and diabetic retinopathy. However, it’s crucial that we catch and treat CSCR, since it often strikes patients in the prime of their lives.
Here, we’ll share the diagnostic cues to watch out for and the treatment approaches that often yield the best outcomes.
Classification of CSCR
Central serous chorioretinopathy is characterized by detachment of the neurosensory retina, secondary to the presence of serous subretinal fluid (SRF).1 Other features of this disease include pigment epithelial detachments (PED) and retinal pigment epithelial changes. It affects men up to five times more than women and is widely regarded as the fourth most common retinal disease after age-related macular degeneration, diabetic maculopathy and vein occlusion.2,3 Patients suffering from CSCR experience distortion of central vision, scotomas, micropsia and metamorphopsia.1 While “central” refers to visual symptoms due to serous detachments in the macula, CSCR can also present with extra-macular involvements that might be asymptomatic.4 It can have a significant impact on quality of life, as it typically affects patients of working age, between 30 and 50 years.3
Despite advancements in medical science, our understanding of CSCR remains incomplete. Its underlying mechanisms involve venous overload and permeability, scleral rigidity and RPE health.3,5 Additionally, both glucocorticoids and mineralocorticoids have been implicated in its pathogenesis. Increasing evidence also suggests CSCR is part of the broader pachychoroid disease spectrum (PDS), further highlighting the condition’s complexity.6
While there’s a lack of standardization among retina specialists over the classification of CSCR, the CSCR International Group proposed to classify CSCR into simple, complex and atypical forms based on multimodal imaging findings.7 The group established a threshold based on a 2-disc area of RPE atrophy to distinguish between simple and complex CSCR: <2 DA is simple; > 2 DA is complex. Each group was then further categorized into: primary (representing the initial episode of SRF); recurrent (indicating the presence of SRF with history or signs of previous episodes); and resolved. SRF persisting for more than six months was classified as persistent. Both groups could be complicated by the presence of choroidal neovascularisation. Additionally, the atypical category encompasses variants such as bullous CSCR, RPE tears or CSCR occurring in conjunction with other retinal diseases.
Although this is a consensus-based classification, it still needs further refinement from the global ophthalmology community, given that the inter-rater agreement still ranges between fair and moderate.8
Other Classifications
Clinicians might be more familiar with the common classification of acute or chronic CSCR, based on the duration of the subretinal detachments: Eyes with duration of less than three to six months are classified as acute, while eyes with duration of more than three to six months are classified as chronic.3,7 In clinical practice, variations in the presentation and progression of disease often result in poor agreement among retina specialists.7 There might also be recall bias or unreliability in the reporting of symptoms by patients. For the purpose of this article, we will use the term acute and chronic as a signifier of duration, but not for the purpose of classification.
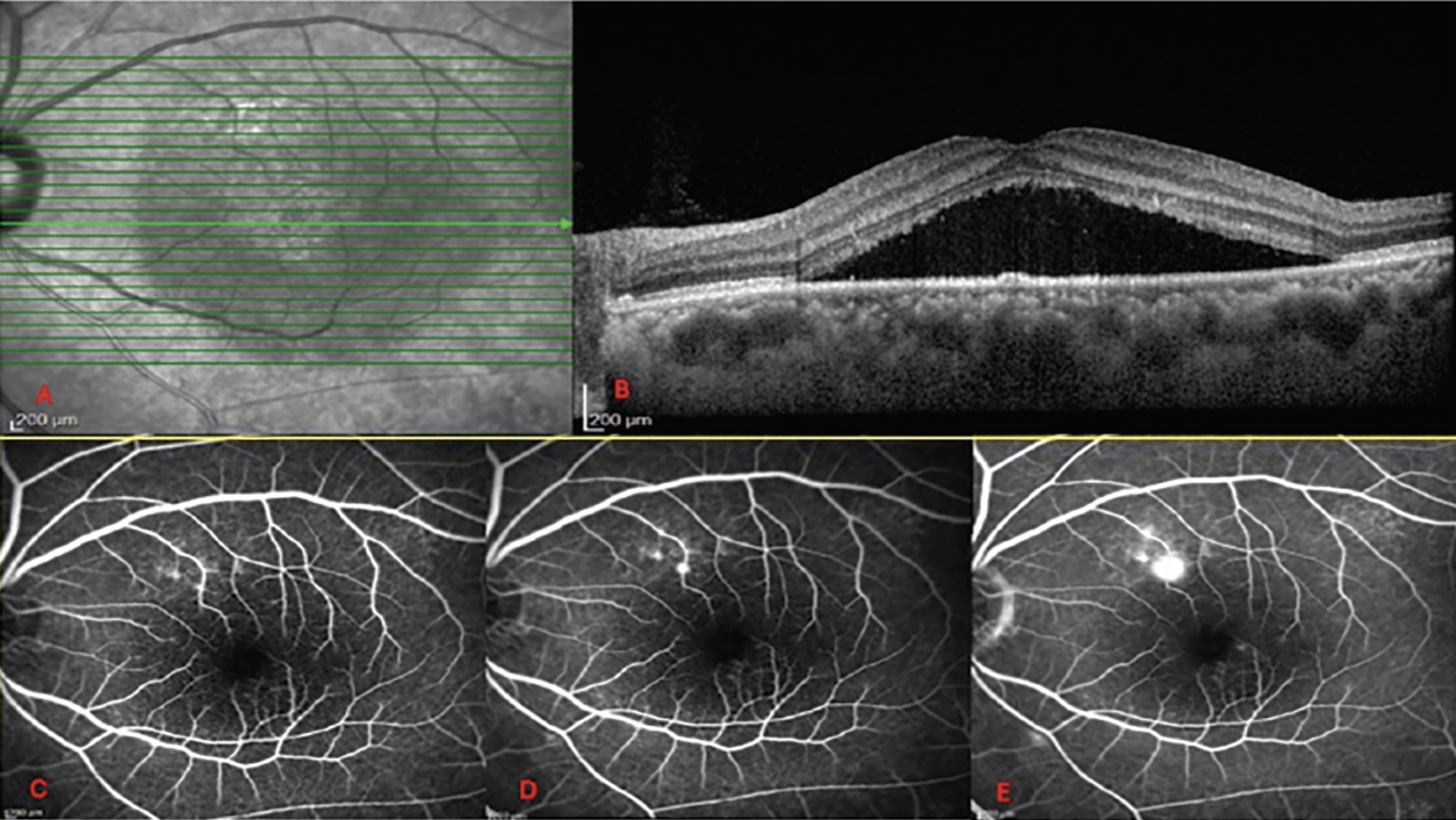 |
|
Figure 1. (A) En face infrared image on OCT scan. (B) Simple CSCR with subretinal fluid and minimal RPE changes. (C, D, E) Early, mid, and late phase of FA, demonstrating an ink blot type lesion which is the source of leakage through the RPE into the subretinal space. |
Diagnosis of CSCR
A detailed medical history is important for determining the nature of symptoms, timeline of disease, and identification of risk factors such as steroid use and other co-morbidities like Cushing’s disease. Questions should include whether this is a first known episode or a recurrent one, as well as the duration of each episode.
A thorough and structured slit-lamp examination would help with the differential diagnosis of other diseases that may also present with similar symptoms and subretinal fluid, such as neovascular diseases, inflammatory diseases like Vogt-Koyanagi Harada syndrome (VKH), optic disc pits and retinal detachments.9 Typical fundoscopy findings in CSCR include serous neurosensory detachment (generally round or oval), with yellow subretinal deposits within the vicinity of the detachment. Additional indicators are RPE pigmentary changes and atrophy.
Following this, the use of multimodal imaging such as optical coherence tomography, fluorescein angiography, indocyanine green angiography and fundus autofluorescence will play a further role in specifying the classification into simple, complex or atypical CSCR.
OCT Findings
OCT is recommended as the primary imaging tool for diagnosing CSCR. It’s useful at detecting various pathologies, including SRF, subretinal fibrin and abnormalities of the RPE such as irregularity, atrophy and detachments.3,10 Furthermore, it’s invaluable for assessing the extent and location of SRF, facilitating comparisons over time. Enhanced-depth OCT can demonstrate choroidal features indicative of pachychoroid such as enlarged vessels in the Haller’s layer, along with thinned choriocapillaris and Sattler’s layer.11
In acute CSCR, serous retinal detachments are typically confined to the macula and exhibit fewer RPE abnormalities. In contrast, chronicity of CSCR can lead to various findings, including elongated photoreceptor outer segments (POS), subretinal fibrin, intraretinal lipid deposits, subretinal yellowish dots, thinning of the outer nuclear layer and widespread RPE changes.3,11–14 Intraretinal fluid can also develop when defects in the external limiting membrane allow fluid to enter the retina.15,16
Chronic neurosensory retinal detachments are generally shallow and broad, with attenuation of the outer retinal layers. Notably, the presence of a morphological feature known as the “Fuji Sign,” characterized by a more peaked appearance of SRF, has been shown to predict a higher likelihood of spontaneous SRF resolution.17
When evaluating OCT images, examining the status of the photoreceptors is crucial for prognostication.13 Disruption of the external limiting membrane (ELM) and/or the ellipsoid zone (EZ) band have been associated with poor central vision, even after the resolution of SRF. Elongation of the POS at baseline is also associated with poorer long term visual acuity outcomes.18
The presence of a PED warrants careful observations of RPE defect within the PED, which can correspond precisely to leakage points on FA.19 RPE changes, such as an RPE bulge have also been noted to occur within areas of choroidal hypermeability.20
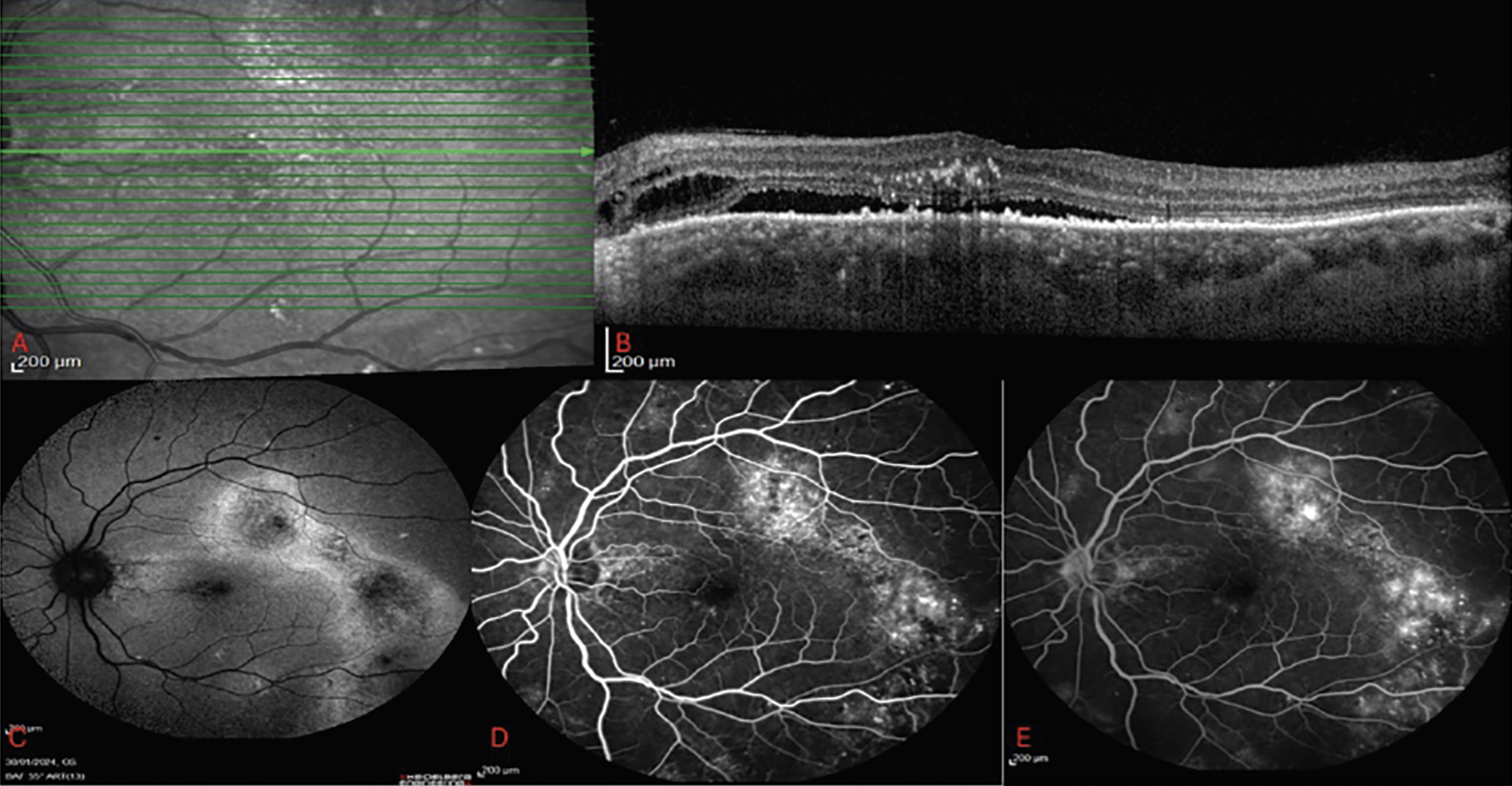 |
|
Figure 2. (A) En-face infrared image on OCT scan. (B) Complex CSCR with subretinal fluid, intraretinal lipid, intraretinal fluid and extensive RPE changes. (C) FAF with heterogenous pattern of hyperautofluorescence and gravitational track changes. (D) Early phase FA with window defect obscuring areas of leakage. (E) Late phase FA demonstrating areas of increased hyperfluorescence corresponding to areas of leakage. |
Fluorescein Findings
On FA, the classic presentation is between one to three focal leakage points, corresponding to areas of RPE defects. It can present with the characteristic “inkblot” leakage: A focal leak appears during dye transit and becomes more indistinct as the dye leaks more slowly into the subretinal space through the RPE defects (Figure 1).11 Another classic pattern on FA is the “smokestack” leakage: a focal hyperfluorescent pinpoint with a spreading area of hyperfluorescence over time.4,21 Fluorescein subsequently pools in the area of neurosensory detachment, resulting in an area of diffuse circular hyperfluorescence.4
In chronic CSCR, gravitational tracts can be present: areas of hyperfluorescent RPE window defects which correspond to RPE atrophy (Figure 2).10 These window defects can make it difficult to identify the focal leakage points as both are hyperfluorescent.
ICGA Findings
On ICGA, signs in the early phase (one to three minutes) include localized delay and uneven filling of the arteries and choriocapillaris.22,23 In the mid-phase (three to 15 minutes), there can be areas of indistinct hyperfluorescence which are a sign of choroidal hyperpermeability.21 These areas correspond to findings of focal leakage on FA. Areas of hyperfluorescence on ICGA are generally more widespread compared to FA.
FAF Findings
In the stage of disease without significant RPE damage, areas of SRF can initially present with hyperautofluorescence.24 Subsequently, when RPE becomes atrophic, this appears as areas of hypoautofluorescence; initially, the patterns appear granular, before progressing and becoming confluent.25,26 However, accumulation of debris from photoreceptor outer segments which persist in the subretinal space can manifest as hyperautofluorescence, which highlights the contextual importance of multi-modal imaging.20
One of the main advantages of FAF is that it’s a convenient and non-invasive imaging tool to visualize RPE and outer retinal changes. In general, eyes with acute CSCR tend to present with homogenous hyperautofluorescene with minimal changes around the area of neurosensory detachment. In contrast, patients with a more chronic presentation can have a more heterogenous pattern of hyperautofluorescence, with more extensive areas of RPE abnormalities (Figure 2).25 This visualization is important for classifying patients into either simple or complex CSCR based on the extent of RPE changes, as well as for the detection of previous areas of extramacular involvement which might have been asymptomatic.
Treatment of CSCR
In the management of CSCR, the primary goal is to achieve the resolution of SRF while maintaining the integrity of the neurosensory retina. The initial approach involves conducting a thorough patient history and identifying and adjusting modifiable risk factors. This includes advising on the cessation or reduction of corticosteroid use, in consultation with other health-care providers, to determine the viable dosage. It’s noted that a considerable proportion of acute CSCR cases can resolve on their own, and even up to 30 percent of chronic CSCR may improve without any intervention. If interventions are required, treatment options include photodynamic therapy, subthreshold laser, anti-VEGF and other potential oral therapies.
Modifiable Risk Factors
One of the most well-known risk factors is corticosteroids; both naturally occurring and medically prescribed corticosteroids, including those administered locally or systematically, have been linked to an increased risk of CSCR.10 Systemic corticosteroids in particular are recognized as an independent risk factor, and are associated with not just onset but also with the prolongation and recurrence of CSCR. Despite the broad use of corticosteroids in medical practice, CSCR remains relatively rare, which suggests that the increased risk may not be strictly dose dependent, but rather influenced by increased vulnerability in certain individuals.
Cushing’s syndrome, characterized by excessive cortisol production, is linked to an increased risk of CSCR. CSCR can sometimes be the presenting feature of patients with Cushing’s syndrome. In one study, up to 7.7 percent of patients with Cushing’s syndrome also had CSCR.28 Furthermore, a case-series report found that SRF can dissipate in CSCR patients following surgical treatment of
Cushing’s syndrome.29
Stress-inducing life situations such as shift work, inadequate sleep, circadian rhythm disruption, as well as type A behavioral traits are all also linked to increased risk of CSCR.30–33 Lastly, CSCR has also been reported to be at an increased risk during pregnancy due to changes in the choroidal circulation, specifically more so in the third trimester.34,35
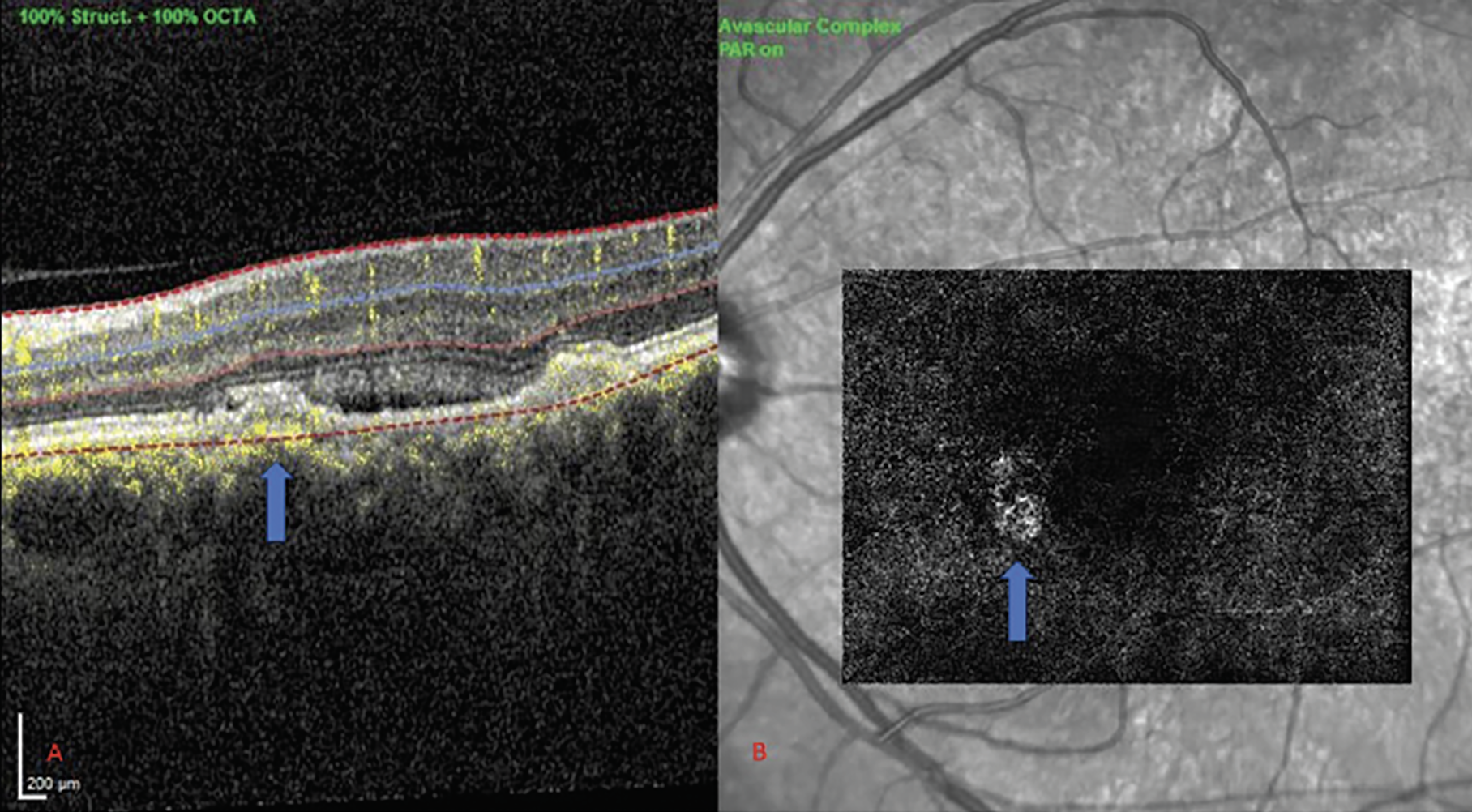 |
|
Figure 3. (A) OCT showing irregular PED in CSCR. (B) CNV corresponding to area of irregular PED (solid blue arrows). |
Non-modifiable Risk Factors
Several risk factors have been identified for central serous chorioretinopathy, this includes male sex, which significantly increases the risk compared to females, as well as age, particularly in the 35- to 44-year-old range.10 Short axial length is another positive association.36
Photodynamic Therapy
PDT involves intravenous administration of the photosensitizing agent verteporfin, followed by targeted application of laser to the area of interest, to produce free radicals which primarily affect the choriocapillaris. This leads to restructuring of vessels within the capillary bed in the vascular endothelium.3 Standard PDT treatment is a dose of 6 mg/m2 body surface area of verteporfin. PDT can also be given at half that dosage.
Similarly, full-fluence PDT is when a light at 689 nm is applied to a designated area with a fluence of 50 J/cm2 for 83 seconds, while half-fluence PDT is 25 J/cm2 with the same duration. Different combinations of PDT dose and fluence are used in different clinical practice, such as full-dose, half-fluence; or half-dose, full-fluence.3 Many groups have reported a high rate of resolution of SRF in CSCR following PDT. In addition, the choroidal thickness and hyperpermeability were also found to be alleviated following PDT.
The area targeted by PDT is guided by FA and ICG. On FA, the target area is the spots of leakage; on ICG, the target area is often set so that the diameter covers the localized hyperfluorescent area during the mid-phase.3
More recent prospective, randomized, controlled studies supporting its use came from the SPECTRA and PLACE trials.37,38 The SPECTRA trial showed that at three months follow-up, 78 percent of patients in the half-dose PDT arm had complete resolution of SRF compared to 17 percent in the eplerenone group.
In the PLACE trial, half-dose photodynamic therapy was compared to high density subthreshold micropulse laser (HSML): treatment with half-dose photodynamic therapy was shown to be superior to HSML in both the focal leakage group and diffuse leakage group in terms of subretinal fluid resolution, at both three months (48 percent vs 16 percent) and 12 months follow-up (67 percent vs 21 percent).
Focal Laser
Focal laser is an inexpensive treatment modality that may be able to address extrafoveal leakage points in CSCR. Focal application of thermal laser was one of the earlier investigated treatment modalities, which is thought to address leakage by inducing scarring of abnormal RPE cells. While previous studies have shown that laser photocoagulation may reduce the duration of CSC, there have been limited trials as focal laser is rarely performed on leakage within 500 um of the fovea due to scarring and possible lesion expansion.39 Focal laser can be a potential option especially for extramacular lesions.
Subthreshold Laser
Subthreshold/micropulse laser involves applying short, subthreshold micropulses of energy to the retina, promoting tissue repair without corresponding retinal damage. Due to its minimal thermal damage, it can be used close to the fovea.3 Various studies have suggested a broad range of micropulse laser techniques and laser types, complicating the comparison across different research findings. Nonetheless, the PACORES trial, although limited by its retrospective study design, compared micropulse laser with half-dose PDT. Although comparisons between the two cohorts weren’t possible due to the study design, both groups demonstrated reduction in central macular thickness at 12 months follow-up.40 In the PLACE trial, the high-density subthreshold micropulse laser (HSML) group achieved resolution of SRF in 28.8 percent of patients.38
Due to its favorable safety profile, the Subthreshold Laser Ophthalmic Society recommend the use of subthreshold laser in one month even for acute CSCR, instead of the convention of observing for a period of three to four months.41 The recommended settings are 5-percent duty cycle, 200 ms pulse duration, 100 to 200 µm spot size, with no spacing between the spots. Some clinicians have argued that the lack of standardization has hindered the more widespread adoption of subthreshold laser.42
Mineralcorticoid Antagonists
Mineralocorticoid dysfunction has been implicated in the pathogenesis of CSCR.43 There have been animal models showing that overexpression of human mineralocorticoid can exhibit characteristics of pachychoroid phenotypes.44 The mineralocorticoid receptor antagonist eplerenone has been long used as a treatment for CSCR. However, the VICI trial didn’t find any significant difference between either 25 mg/day oral eplerenone, increasing up to 50 mg/day versus placebo. Nevertheless, one of the arguments against the findings is that eplerenone should be continued even where there is resolution of SRF, as stopping treatment can result in reoccurrence.45
While multiple other oral medication treatment has been described, supportive evidence has been limited to mostly uncontrolled case series.
Anti-VEGF
Some case series have reported resolution of SRF in CSCR following treatment with anti-VEGF. However, the role of VEGF in the pathogenesis of CSCR is unclear. More recently, the incorporation of OCTA in the work-up of CSCR has helped to identify that secondary CNV may complicate CSCR in 24 to 39 percent of patients.46,47 Type 1 neovascularization occurs sub-RPE and can present with a shallow irregular PED or flat irregular PED (FIPED) (Figure 3).48,49 It’s now believed that this group of eyes are likely to respond to intravitreal anti-VEGF therapy.37 A case series of 88 patients with chronic CSCR identified neovascularization in one-third of eyes with shallow irregular PEDs on OCTA.50 Therefore, eyes with these features should be evaluated in further detail with OCTA.
For eyes with CSCR without CNV, there’s no substantial evidence indicating benefits in terms of anatomical or visual acuity results.
It’s also important to consider type 1 neovascularization as part of pachychoroid neovasculopathy. Clues that point to PNV are Type 1 neovascularization lesion with pachychoroid features, thickened choroid, absent soft drusen and RPE changes overlying pachyvessels. PNV can be difficult to distinguish from CSCR on FA, as both can have quite similar angiographic signs. However, CSCR eyes are more likely to have the characteristic changes on FAF, such as descending tracts.
The Pachychoroid Disease Spectrum
The clinical relevance of pachychoroid in the context of CSCR lies in its inclusion within the pachychoroid disease spectrum (PDS), which encompasses a number of clinical conditions that share similar abnormalities in the choroid.11 PDS conditions can evolve from one to another. Pachychoroid pigment epitheliopathy is considered a forme fruste of CSCR, but SRF may develop in these eyes during longitudinal follow-up. Choroidal congestion in PDS may aggravate or perpetuate choriocapillaris impairment. The resulting ischemic environment has been proposed to promote neovascularization in the form of pachychoroid neovasculopathy or polypoidal choroidal vasculopathy. Hence eyes with CSCR may develop secondary CNV within this context. Additionally, variations in the CFH gene have been linked to differences in choroidal thickness among specific Asian populations.51
In conclusion, our current understanding of CSCR has led to a new classification consensus. The adoption of multimodal imaging allows for more precise evaluation of the extent of RPE involvement, as well as identifying the areas of leakage. Current best clinical practice involves careful patient assessment, modification of risk factors and judicious use of different treatment options based on availability and clinical presentation.
Dr. Chong and Dr. Gilead are both senior clinical research fellows at the Singapore Eye Research Institute.
Dr. Cheung is currently a professor at Duke-NUS Medical School, National University of Singapore (NUS). She is the head and senior consultant of the Medical Retina Department at SNEC, and head of the Retina Research Group at the Singapore Eye Research Institute.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
1. Daruich A, Matet A, Behar-Cohen F. Central serous chorioretinopathy. Macular Edema 2017;58:27–38.
2. Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology 2008 Jan;115:1:169–73.
3. Feenstra HMA, van Dijk EHC, Cheung CMG, Ohno-Matsui K, Lai TYY, Koizumi H, et al. Central serous chorioretinopathy: An evidence-based treatment guideline. Prog Retin Eye Res 2024 Jan 31 [online ahead of print];101236.
4. Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res 2015;48:82–118.
5. Spaide RF, Gemmy Cheung CM, Matsumoto H, Kishi S, Boon CJF, van Dijk EHC, et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 2022 Jan;86:100973.
6. Brown R, Mohan S, Chhablani J. Pachychoroid spectrum disorders: An updated review. J Ophthalmic Vis Res 2023;18:2:212–29.
7. Chhablani J, Cohen FB, Aymard P, Beydoun T, Bousquet E, Cohen FB, et al. Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retina 2020 Nov 1;4:11:1043–6.
8. Chhablani J, Behar-Cohen F, Central Serous Chorioretinopathy International Group. Validation of central serous chorioretinopathy multimodal imaging-based classification system. Graefes Arch Clin Exp Ophthalmol 2022;260:4:1161–9.
9. van Dijk EHC, Boon CJF. Serous business: Delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog Retin Eye Res 2021 Sep;84:100955.
10. Kaye R, Chandra S, Sheth J, Boon CJF, Sivaprasad S, Lotery A. Central serous chorioretinopathy: An update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res 2020 Nov;79:100865.
11. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye Lond Engl 2019;33:1:14–33.
12. Spaide RF, Klancnik JM. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005;112:5:825–33.
13. Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol 2008;145:1:162–8.
14. Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol 2009;148:1:105-110.e1.
15. Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina Phila Pa 2003;23:1:1–7;quiz 137–8.
16. Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina Phila Pa 2003;23:6:752–63.
17. Feenstra HMA, Hensman J, Gkika T, Lipkova V, Hoyng CB, Diederen RMH, et al. Spontaneous resolution of chronic central serous chorioretinopathy: ‘Fuji Sign’. Ophthalmol Retina 2022;6:9:861–3.
18. Asano KS, Asaoka R, Asano S, Azuma K, Inoue T, Obata R. Elongated photoreceptor outer segment length and prognosis of chronic central serous chorioretinopathy. Retina Phila Pa 2020;40:4:750–7.
19. Fujimoto H, Gomi F, Wakabayashi T, Sawa M, Tsujikawa M, Tano Y. Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-domain optical coherence tomography. Ophthalmology 2008;115:9:1494–500, 1500.e1-2.
20. Hirami Y, Tsujikawa A, Sasahara M, Gotoh N, Tamura H, Otani A, et al. Alterations of retinal pigment epithelium in central serous chorioretinopathy. Clin Experiment Ophthalmol 2007;35:3:225–30.
21. Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol (Copenh) 2008;86:2:126–45.
22. Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996 Jan;121:1:26–34.
23. Pang CE, Shah VP, Sarraf D, Freund KB. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol 2014;158:2:362-371.e2.
24. Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: An update on pathogenesis and treatment. Eye 2010;24:12:1743–56.
25. Lee WJ, Lee JH, Lee BR. Fundus autofluorescence imaging patterns in central serous chorioretinopathy according to chronicity. Eye Lond Engl 2016;30:10:1336–42.
26. Zola M, Chatziralli I, Menon D, Schwartz R, Hykin P, Sivaprasad S. Evolution of fundus autofluorescence patterns over time in patients with chronic central serous chorioretinopathy. Acta Ophthalmol (Copenh) 2018;96:7:e835–9.
27. Spaide R. Autofluorescence from the outer retina and subretinal space: Hypothesis and review. Retina Phila Pa 2008;28:1:5–35.
28. Holtz JK, Larsson JME, Hansen MS, van Dijk EHC, Subhi Y. Pachychoroid spectrum diseases in patients with Cushing’s syndrome: A systematic review with meta-analyses. J Clin Med 2022;29;11:15:4437.
29. van Dijk EHC, Dijkman G, Biermasz NR, van Haalen FM, Pereira AM, Boon CJF. Chronic central serous chorioretinopathy as a presenting symptom of Cushing syndrome. Eur J Ophthalmol 2016;26:5:442–8.
30. Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Retina Phila Pa 2012;32 Suppl 1:709.
31. Ji Y, Li M, Zhang X, Peng Y, Wen F. Poor sleep quality is the risk factor for central serous chorioretinopathy. J Ophthalmol 2018;2018:9450297.
32. Setrouk E, Hubault B, Vankemmel F, Zambrowski O, Nazeyrollas P, Delemer B, et al. Circadian disturbance and idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2016;254:11:2175–81.
33. Bousquet E, Dhundass M, Lehmann M, Rothschild PR, Bayon V, Leger D, et al. Shift work: A risk factor for central serous chorioretinopathy. Am J Ophthalmol 2016;165:23–8.
34. Sunness JS. The pregnant woman’s eye. Surv Ophthalmol 1988;32:4:219–38.
35. Yu J, Li L, Jiang C, Chang Q, Xu G. Clinical characteristics of pregnancy-associated central serous chorioretinopathy in the Chinese Population. J Ophthalmol. 2021 Dec 16;2021:e5580075.
36. Terao N, Koizumi H, Kojima K, Kusada N, Nagata K, Yamagishi T, et al. Short axial length and hyperopic refractive error are risk factors of central serous chorioretinopathy. Br J Ophthalmol 2020;104:9:1260–5.
37. van Rijssen TJ, van Dijk EHC, Tsonaka R, Feenstra HMA, Dijkman G, Peters PJH, et al. Half-dose photodynamic therapy versus eplerenone in chronic central serous chorioretinopathy (SPECTRA): A randomized controlled trial. Am J Ophthalmol 2022;233:101–10.
38. van Dijk EHC, Fauser S, Breukink MB, Blanco-Garavito R, Groenewoud JMM, Keunen JEE, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: The PLACE Trial. Ophthalmology 2018;125:10:1547–55.
39. Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1979;63:10:674–7.
40. Roca JA, Wu L, Fromow-Guerra J, Rodríguez FJ, Berrocal MH, Rojas S, et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: Results of the Pan-American Collaborative Retina Study (PACORES) Group. Br J Ophthalmol 2018;102:12:1696–700.
41. Chhablani J. Subthreshold laser therapy guidelines for retinal diseases. Eye 2022;36:12:2234–5.
42. Keunen JEE, Battaglia-Parodi M, Vujosevic S, Luttrull JK. International Retinal Laser Society guidelines for subthreshold laser treatment. Transl Vis Sci Technol 2020;9:9:15.
43. Bousquet E, Zhao M, Daruich A, Behar-Cohen F. Mineralocorticoid antagonists in the treatment of central serous chorioetinopathy: Review of the pre-clinical and clinical evidence. Exp Eye Res 2019;187:107754.
44. Canonica J, Zhao M, Favez T, Gelizé E, Jonet L, Kowalczuk L, et al. Pathogenic effects of mineralocorticoid pathway activation in retinal pigment epithelium. Int J Mol Sci 2021;22:17:9618.
45. Stanescu-Segall D, Touhami S, Bodaghi B, LeHoang P. Eplerenone for chronic central serous chorioretinopathy. The Lancet 2020;396:10262:1556–7.
46. Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology 2019;126:4:576–88.
47. Savastano MC, Rispoli M, Lumbroso B. The incidence of neovascularization in central serous chorioretinopathy by optical coherence tomography angiography. Retina Phila Pa 2021;41:2:302–8.
48. Bonini Filho MA, de Carlo TE, Ferrara D, Adhi M, Baumal CR, Witkin AJ, et al. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmol 2015;133:8:899–906.
49. Liu T, Lin W, Zhou S, Meng X. Optical coherence tomography angiography of flat irregular pigment epithelial detachments in central serous chorioretinopathy. Br J Ophthalmol [online ahead of print]. 2020 Apr 7 [Accessed Mar 14, 2024]. Available from: https://bjo.bmj.com/content/early/2020/05/05/bjophthalmol-2019-315318
50. Bousquet E, Bonnin S, Mrejen S, Krivosic V, Tadayoni R, Gaudric A. Optical coherence tomography angiography of flat irregular pigment epithelium detachment in chronic central serous chorioretinopathy. Retina Phila Pa 2018;38:3:629–38.
51. Fenner BJ, Li H, Gan ATL, Song YS, Tham YC, Jonas JB, et al. Genetic variability of complement factor H has ethnicity-specific associations with choroidal thickness. Invest Ophthalmol Vis Sci 2023;64:2:10.