Yet, evidence suggests that the cloud of material and gases created by every laser pulse may affect visual outcomes and could have health consequences for those who breathe it in. Here, we'd like to profile three instruments designed to address this issue at the source, and review some of the evidence that removing laser plume is important.
Why Evacuate the Plume?
The first people to develop systems to remove excimer laser plume were, in fact, the first people to use excimer lasers. Excimer lasers were originally designed to etch computer chips, and it became clear early on that the plume of smoke rising from the chips during ablation interfered with the laser beam, causing unacceptable inaccuracies in the ablation. These problems were resolved with the addition of laser-plume evacuators.
 |
| Laser plume rises from the eye during LASIK surgery. Leon C. LaHaye, MD |
Today, you can see sparkles in the air over the patient's eye during LASIK, for the same reason: The laser beam is striking particles in the cloud of laser plume. Given the improvement in computer chip etching caused by removing the plume, doing so in refractive surgery—where someone's vision is at stake—seems like a reasonable option to consider.
The second concern associated with the escape of laser plume into the OR environment is the potential for negative effects on the health of the individuals who inhale it. Claims that the plume is sterile and/or harmless may be unwarranted, and many devices that seem to offer protection may not be effective. A surgical mask, for example, is designed to protect the patient from spittle, not to protect the wearer from airborne particles or gases. (For more on health concerns, see "Is Your Health at Risk?" on page 38.)
In addition, removing the plume minimizes or eliminates the odor that accompanies laser ablation of corneal tissue. This can make the surgery far more pleasant for the surgeon and staff, and less worrisome for the patient.
The Evidence for Better Outcomes
Many surgeons have reported studies conducted in their practices (some published, some not) comparing outcomes when LASIK is performed with and without plume evacuation:
• In a study published in 2002, Keith Charles, MD, retrospectively analyzed three-month LASIK postoperative data for 117 eyes treated with a laser plume evacuator and 82 eyes treated without an evacuator.1 The laser-plume evacuator reduced the incidence of diffuse lamellar keratitis following LASIK from 1.2 percent to zero. He also noted a statistically significant difference in postoperative residual refractive error and uncorrected visual acuity between the two groups (p<0.001). For example, in the group treated with a plume evacuator, 92 percent were within ±0.50 D of the intended correction; in the group treated without a plume evacuator, only 65 percent were.
• At the 2001 ASCRS meeting, Steven J. Dell, MD, director of refractive and corneal surgery at Texan Eye Care in Austin, Texas, reported an unpublished retrospective study of 100 consecutive cases, half performed with plume evacuation, half without. Four percent of eyes ablated without plume removal developed DLK; with plume evacuation, no eyes developed DLK. (These results later inspired Dr. Dell to create his own plume evacuation system, profiled below.)
• Stephen S. Dudley, MD, FACS, conducted a study of 42 eyes in his practice; half received LASIK with a Visx Star S2 laser while using the Mastel Clean Room System, half without. Before surgery, the two groups had no statistical difference in mean SE, but the difference postop was statistically significant (p=0.02). At two weeks postop, 90 percent of the group with plume removal had UCVA of 20/30 or better; only 81 percent of the controls did. In the plume removal group, 57 percent were 20/20 or better; in the control group, only 33 percent were.
• Robert Gale Martin, MD, of Carolina Eye Associates in Southern Pines, N.C., tracked the outcomes in 42 eyes of his patients, half receiving LASIK with the Mastel Clean Room, half without. At two weeks postop, 57 percent of the group undergoing LASIK with plume evacuation were 20/20 or better; only 33 percent of the control group achieved the same result.
Many surgeons who have used the Mastel Clean Room for several years have reported noting a difference in their patients' visual outcomes and a reduction in complications and enhancements. James Lewis, MD, a surgeon at Wills Eye Hospital and Surgical Network and director of refractive surgery at the Pennsylvania College of Optometry, says using the Mastel plume evacuator for the past eight years has stabilized his surgical outcomes and lowered his enhancement rate. Similarly, Kurt A. Buzard, MD, FACS, who practices in Las Vegas, has reported that using the Mastel Clean Room System resulted in an average increase of one to two Snellen lines and superior vision quality for his patients.
Dr. Dell notes that clearing away the plume can increase the amount of ablation significantly. "Using the PlumeSafe evacuator with the Visx S2, S3, and Alcon lasers causes a 10-percent additional ablation effect," he says. "At a rate of 15 to 20 liters of air per minute, the degree of added effect might be one-quarter diopter of change or less; not enough to necessitate changing your nomogram." However, at higher flow rates, he notes, adjusting your nomogram becomes important.
Handheld Plume Evacuators
Currently, several laser manufacturers have acknowledged the potential importance of plume removal by incorporating vacuum devices into their lasers. However, their relatively large size limits how close they can be positioned to the source of plume during surgery.
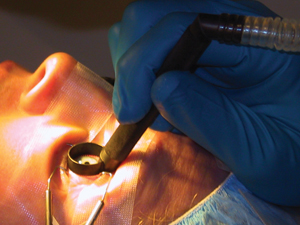 |
| Plume evacuators like the Dell PlumeSafe System (pictured above) also help control hydration, prevent contamination and stabilize the eye. |
Three handheld plume evacuation devices offer an alternative: They use a fixation ring to locate the evacuation ports extremely close to the corneal surface, creating a partially enclosed "microclimate," and making plume removal highly efficient. The ring also helps prevent unwanted sources of debris and bacteria from entering the surgical area, and makes it possible to control the hydration of the cornea to a far greater extent. In addition, the fixation ring stabilizes the globe during LASIK, helping to minimize saccadic movement and control cyclotorsion.
The Dell PlumeSafe System
The Dell PlumeSafe Evacuation System, designed by Dr. Dell (manufactured by Buffalo Filter and distributed by ASICO) consists of a plume aspiration fixation ring handpiece connected to a control unit that runs the evacuate through an ultra-low penetration air filter and an activated charcoal layer designed to absorb polyaromatic hydrocarbons. Features of the PlumeSafe System include:
• a microflow adjustment that lets you control the aspiration flow rate over a range of 10 to 60 liters per minute. The flow rate is displayed in a digital readout. "This is very important," notes Dr. Dell, "because as the filter loads with debris, the flow rate of a nonadjustable system will drop, lowering the efficacy of the evacuation and the effect on corneal hydration. You can adjust the PlumeSafe system to keep the flow rate constant."
• an LED indicator, showing how long the filter has been in use.
• a low maintenance brushless motor.
• "whisper technology" to minimize operational noise.
The handpiece is coated with an infrared-absorbing coating designed to minimize interference with eye trackers. "We've used the system successfully with the Visx, Alcon and LaserSight lasers, and I know surgeons have used it with the Nidek laser," he says.
When laser plume is not contained, individuals present at the surgery are virtually certain to inhale some of the particles and gases being produced. The anecdotal evidence of health trouble—some mild, some serious—is plentiful. Clinical studies, however, have been relatively few. Nevertheless, the existing data does provide reason for concern. Elements that have been identified in laser plume include the gases benzene, hydrogen cyanide, toluene, formaldehyde, and polycyclic aromatic hydrocarbons.1,2 Studies of excimer laser plume smoke have found that live viruses can be isolated from the plume evacuate.3,4,5 Other laser studies have also shown that bacteria, viruses and DNA can survive laser ablation.6,7,8 (The dermatology literature contains multiple references to the health problems resulting from breathing plume smoke emitted during CO2 laser use.) The National Institute for Occupational Safety and Health and the Occupational Safety and Health Administration have both stated that plume smoke should be evacuated and treated as infectious waste. Some of the particles created are between 1.0 and 0.4 µm,9 the same size range as coal or asbestos dust, making the particles invisible and lighter than air. Unless contained, they remain floating in the air, as coal dust does. In addition, excimer lasers are often calibrated by vaporizing test plates made of materials such as vinyl, polymethylmethacrylate and aluminum mylar; the consequences of breathing the laser plume from these materials is not known. About a year and a half ago, Matthew Niemeyer, MD, a resident in ophthalmology under Howard V. Gimbel, MD, at Loma Linda University, conducted a survey of ophthalmology personnel in the United States designed to look at the effects of laser plume on the health of those who are regularly exposed to it. About 250 surveys (out of 500) were completed and returned. The control group (about 125 respondents) consisted of ophthalmologists and technicians who work in the same clinic or building as the people using excimer lasers, but do not use a laser as part of their surgeries or other procedures. The results showed a highly statistically significant difference between the groups; personnel exposed to laser plume had more symptoms as a group than other doctors working in the same buildings. Those who were exposed to the plume reported a significantly greater amount of runny nose, cough and allergy problems—primarily respiratory symptoms. (Differences in more serious conditions such as autoimmune disease were not significant.) Like any good scientist, most ophthalmologists prefer to take action only when clinical evidence is substantial and compelling. However, long-term health risks can take years to establish clinically, by which point considerable damage may have been done.
1. Kahle G, Stadter H, Seiler T, Wollensak J. Gas chromatographic and mass spectroscopic analysis of excimer and erbium:Yttrium aluminum garnet laser-ablated human cornea. Invest. Ophthalmol Vis Sci 1992;33:2180-2184. 2. Randolph D. Glickman, Yun Liua, George L. Mayoa, Alan D. Baribeaub, Tomy Starckc, Tom Bankheadd. Composition of the excimer laser-induced plume produced during LASIK refractive surgery. Presentation at Photonics West Conference, International Society for Optical Engineering, January 2003. 3. Taravella MJ, Weinberg A, May M, Stepp P. Live virus survives excimer laser ablation. Ophthalmology 1999;106:1498-1499. 4. Moreira L, Sanchez D, Trousdale M, Stevenson D, Yarber F, McDonnell P. Aerosolization of infectious virus by excimer laser. Am J Ophthalmol 1997;123:297-302. 5. Ziegler B, Thomas C, Meier T, Muller R, Fliedner T, Weber L. Generation of infectious retrovirus aerosol through medical laser irradiation. Las Surg Med 1998:22:37-41. 6. McKinley IB Jr, Ludlow MO. Hazards of laser smoke during endodontic therapy. J Endod 1994 Nov;20:11:558-9. 7. Garden JM, O'Banion MK, Shelnitz LS, Pinski KS, Bakus AD, Reichmann ME, Sundberg JP. Papillomavirus in the Vapor of Carbon Dioxide Laser-Treated Verrucae. JAMA 1988 Feb 26;259:8:1199-202. 8. Garden JM, O'Banion MK, Bakus AD, Olson C. Viral disease transmitted by laser-generated plume (aerosol). Arch Dermatol 2002 Oct;138(10):1303-7. |
The LaFaci Surgical System
This instrument, created by Leon C. LaHaye II, MD, who practices in Lafayette, La., is a multifunction handpiece designed to perform 11 specialized functions in the surgical field once the flap has been made and is ready to be lifted. LaFaci, which short for "LASIK facilitator," is more expensive than the Dell PlumeSafe device, but is designed to serve more purposes during surgery.
The LaFaci System is placed on the eye before you lift the flap and remains in position until after wound closure. Plume evacuation is accomplished through seven ports in the fixation ring which create an adjustable-rate lamellar flow of air across the stromal surface. Like the other handheld options, the device downsizes and contains the surgical field, provides ocular fixation (that is compatible with most laser tracking systems) and makes it easy to manage stromal bed hydration. However, the LaFaci System also provides irrigation and aspiration and manages temporary flap placement and flap repositioning.
 |
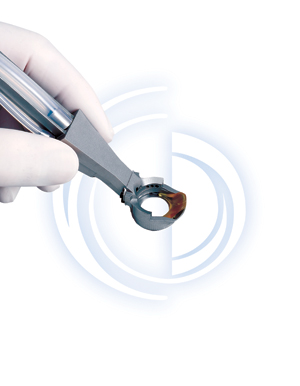 |
| The LaFaci Surgical System performs multiple functions during LASIK, to help standardize the procedure and produce more predictable outcomes. |
"The LaFaci instrument is designed to standardize LASIK by eliminating variables from the procedure," explains Dr. LaHaye. "In conventional LASIK our surgical boundaries include the lid margin, the lashes, the conjuntiva, the surgical drape and all the backwash. When you place LaFaci on the eye, you downsize the surgical field to the limbus. This eliminates potential sources of contamination or unwanted hydration.
"The LaFaci system also lets you use as much irrigation as you need, at any time during the procedure, without having to worry about backwash contamination," he notes. "I hit the footswitch and it delivers a lamina flow of irrigation right across the cornea."
Like the other handheld options, the LaFaci System lets the surgeon remove any unwanted hydration before or during the ablation without touching the bed, through aeration. "I intermittently cease ablation during the procedure and use the switch in the footpedal to provide a couple of seconds of air. When I see that the target tissue is getting shiny again, I know the tissue hydration has changed."
Dr. LaHaye notes that standard LASIK surgery doesn't manage the flap. "LaFaci has a platform to anatomically support the lifted flap and protect it from contaminants or hydration changes," he says. "After the ablation you can rinse and rehydrate the tissue without having to be concerned about backwash. Then you just flip the platform over and in one motion the flap falls right back into position."
Dr. LaHaye says he believes variability from procedure to procedure is the cause of most enhancements. "Using this instrument, my enhancement rate is under 1 percent and about 90 percent of my patients are within ±0.25 D of intended correction," he says.
The Mastel Clean Room System
The Mastel System was the first handheld plume evacuator system, consisting of a modified Fine-Thornton ring with a barrel top containing four vacuum ports, with adjustable vacuum rate. It uses two filters—an ultralow penetrating 0.1-µm air filter designed to capture airborne particles and a carbon-activated filter to capture aromatic hydrocarbons. It also features a vitrectomy lightpipe positioned on the left side of the ablation tube that allows you to lower the overhead illumination, providing a clearer view of the ablation and making it easier for the patient to focus on the target.
 |
| The Mastel Clean Room System, the first handheld plume evacuator, is temporarily unavailable in the United States. |
The Mastel system is temporarily unavailable. The system was marketed for several years erroneously as a Class One instrument, and the FDA is now requiring the company to submit a 510(K) application for the Clean Room System as a Class Two device. Because no adverse events have been reported and a recall would negatively affect practices already using the instrument, no recall has been required. However, sales and marketing in the United States have been suspended until the new submission is approved.
Because this system was the first laser-plume evacuator available, it has been part of many clinical studies and is still used by many surgeons. Dr. Lewis, who has used the Mastel plume evacuator for about eight years, says, "My staff won't tolerate corneal ablation without Mastel's device."
Doug Mastel, president of Mastel Precision, says he hopes the company will be able to resume marketing the device soon.
For More Information ...
To find out more about the Dell PlumeSafe Evacuation System, call
1 (800) 343-2324 (within the United States). For information about the LaFaci System, visit lafacilasik.com on the Web, or call 1 (888) 400-8844.
1. Charles K. Effects of laser plume evacuation on laser in situ keratomileusis outcomes. J Refract Surg 2002 May-Jun;18:3 Suppl:S340-2.