To the Editor:
I am writing in response to the article in your March issue, "When Fourth Generation Drugs are not Enough" (March 2006, p. 69). I believe that there are some areas in this article which require additional clarification.
At the very beginning, the article questions the definition of fluoroquinolone generations, thereafter grouping levofloxacin in with moxifloxacin and gatifloxacin. I believe it is important to point out that levofloxacin is not in the same class or generation as is gatifloxacin or moxifloxacin, regardless of the classification scheme. There are multiple published opinions that support this position.1,2,3
Additionally, although "superbugs" were referenced heavily throughout the article, they are not well-defined. It is important for clinicians to know whether or not these "superbugs" are methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, multi-drug resistant S. pneumoniae, multi-drug resistant gram negative bacilli, etc. Also on the clarification front, the article does not adequately explain exactly what a "fortified antimicrobial" is. It would be helpful for the readers to know how the antibiotic is fortified and just how this makes the antibiotic more potent. To this point, there is new evidence to suggest that one of the fourth generation fluoroquinolones, gatifloxacin, can act as a fortified antibiotic against MRSA given its additive or synergistic behavior with its preservative, BAK. At ARVO 2006, I was part of a study designed to evaluate the antimicrobial efficacy of gatifloxacin with and without benzalkonium chloride (BAK) compared with moxifloxacin against a plethora of pathogens including S. aureus. (Borsos S, et al. Invest Ophthalmol Vis Sci. 2006;47:ARVO E-Abstract 1892.) In that study, we found that the combination of gatifloxacin and BAK was highly efficacious against a variety of common ocular pathogens in vitro, providing MIC90 values that were eight- to 125-fold lower than the MIC90 values of gatifloxacin or moxifloxacin alone. In a different study I presented at ARVO 2006, we evaluated the antimicrobial efficacy of gatifloxacin with and without BAK and unpreserved moxifloxacin against MRSA. (Blondeau JM, et al. Invest Ophthalmol Vis Sci. 2006;47:ARVO E-Abstract 1903.)
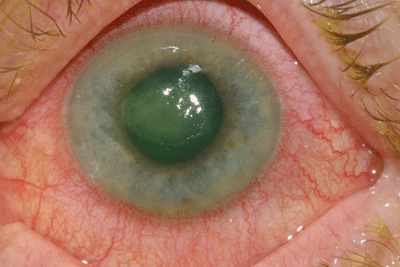
In this study of MRSA clinical isolates in vitro, the combination of gatifloxacin and BAK provided MIC values that were approximately two- to 500-fold better than the MIC values provided by gatifloxacin or moxifloxacin alone. While data was not shown in our ARVO, 2006 presentations, it is worthy of note that similar reductions in MICs were seen in experiments where BAK was tested in vitro with moxifloxacin against both drug-susceptible and resistant bacterial isolates. This data suggests that BAK offers some additive benefit to the formulation as evidenced by the substantial reductions in MICs seen in experiments where BAK was tested along with drug. Although the article states that the fourth generation fluoroquinolones are not good for MRSA eye infections, there is new data to suggest this may not be the case, and further investigations are ongoing.
On a different topic, an example is noted of a patient with a "superbug" being encountered and that the patient was suffering from a community acquired, contact lens related ulcer which hadn't responded to fourth generation fluoroquinolone treatment but responded to a fortified antimicrobial. However, the physician was unable to acquire a positive culture from the patient, stating that the fourth generations do a pretty good job of killing most things. My question is, if the patient is culture negative, which "smartbugs" or "superbugs" are present? Additionally, while I agree that a reasonable and necessary strategy is to change therapy in a patient that does not appear to be clinically responding, there is no way of knowing from the information presented that the patient would not have responded if left on the initial treatment antibiotic—especially since there were no organisms recovered during "treatment failure" with the first drug. The difficulty with these assertions is that there is no supporting clinical trial data to back up the comment that drug A worked better than drug B, as very few or any clinical trial data is available on therapy in patients with resistant organisms. And finally, there is no explanation given for the fortified antimicrobial success in the patients suffering from "smartbugs" but were also culture-negative. The explanation for the success with the change in therapy can only be answered in a well-designed clinical trial in patients with resistant organisms (smartbugs or superbugs) that are treated with different antimicrobials.
In the last paragraph of the article, the suggestion is made that the fourth generation agents should be used empirically first and if success is not attained, the clinician should switch treatment to a fortified agent, culture the patient and try to determine the organism's susceptibility pattern. In my opinion, this is not the optimal approach. In an ideal world, a patient would be cultured prior to the initiation of any antimicrobial agent in order to confirm the pathogen and its susceptibility to various antimicrobial compounds. As a clinical microbiologist, the protocol of waiting to culture until therapeutic failure has occurred seems inefficient and I am well aware of the various arguments for initial empiric therapy without culture and sensitivity data. Unfortunately, in today's environment of multi-drug resistant bacteria, culture prior to therapy seems of paramount importance for optimal patient care.
This article addresses an important issue for clinicians; "smartbugs" or "superbugs" are on the rise and fighting them is difficult. Unfortunately, the articles offers more opinion and personal experience than peer-reviewed data in support of the arguments made. With new, peer-reviewed data on the horizon about fighting "smartbugs" or "superbugs" with our current arsenal of anti-infectives at ARVO, I anticipate that the protocol for treatment of resistant pathogens will evolve as we learn more about the fourth generation fluoroquinolones and other drug classes as well as the bugs they inhibit or kill.
1. Blondeau JM. Fluoroquinolones: Mechanism of action, classification, and development of resistance Surv Ophthalmol 2004;49:S73-S78.
2. Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: An update. Clin. Microbiol Infect 2005;11:256-280.
3. Ball P, Fernald A, Tillotson GS. Therapeutic advances of new fluoroquinolones. Expert Opinions on Investigational Drugs 1998;7:761-783.
Joseph M. Blondeau MSc, PhD
Head, Clinical Microbiology
Royal University Hospital
Adjunct Professor of Microbiology and Immunology
Clinical Assistant Professor of Pathology
University of Saskatchewan, Saskatoon, Saskatchewan
More on Floppy Intraoperative Iris
To the Editor:

I read with interest your article on dealing with Flomax. We reported this problem to the makers of Flomax four years ago. They didn't do anything with the information, but the competition did contact our clinic and initiated an investigation. We have been dealing with this problem for a while. I think I have the best solution for dealing with the problem that was not mentioned in your article, but I have mentioned it to Dr. Chang and Dr. Osher. I prechop the nucleus and take a small nuclear piece and lodge it underneath the incision site. The nuclear fragment keeps the iris from flopping out of the wound and keeps the iris down so you can complete the case. When the rest of the nuclear fragments are removed you inject viscoelastic to keep the rest of the iris down and remove the last piece. I have been doing this for three years with great success. I am glad that Dr. Chang has brought this problem to light. Hopefully this technique will help physicians deal with IFIS.
Rolando Toyos, MD
Memphis, Tenn.
A 0.5-mm Miss Is as Good as a Mile
To the Editor,
I would like to respond to "Keep Your Cataract Surgeries on Target," (May 2006, p 22). I think the optical CCC guidance system being developed by Dr. Perez is ingenious and invaluable. However, I think there may be an error in the article. He states he "generates three aiming rings: one on the limbus; a smaller one that"s the diameter of the IOL, which is 6.0 mm in most cases; and then an even smaller one at 5.5 mm." It appears that the smallest of the three rings in the photo may actually be 5.0 mm in diameter, which means the inner ring would have a 0.5 mm space between it and the adjacent 6.0-mm outer ring on each side. In addition, attempting to generate a CCC of 5.5 mm, in my opinion, is too large for most 6.0-mm IOL optics, as it leaves only a 0.25-mm rim of anterior capsule overlapping the optic edge. Subsequent fibrotic contraction may then lead to adhesion of the anterior capsule to the posterior capsule adjacent to the IOL optic, anteriorly displacing the optic out of the capsular bag, which subsequently can result in increased myopia and accelerated posterior capsular opacification. Therefore, I aim for, and would recommend, a 5.0-mm CCC in most cases in order to have a minimum capsular overlap of 0.5 mm over a 6.0 mm IOL optic.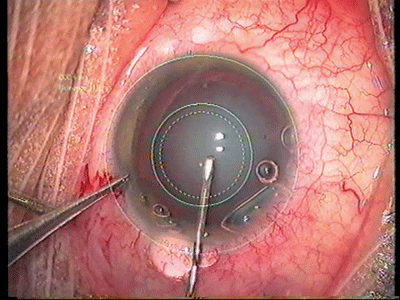
Harry B Grabow, MD
Sarasota Cataract & Laser Institute
Sarasota, Fla.
Editor's note: Sharp-eyed Dr. Grabow is correct; the inner ring is 5 mm in diameter, and the 5.5-mm description was incorrect.
An Anesthesiologist Gets an IOL
To the Editor:
As the associate director of anesthesia at an eye clinic, I have had a great opportunity to observe the various techniques of cataract surgery, especially as relates to anesthesia. Indeed, the topical technique, usually employing tetracaine drops along with intracameral lidocaine, always appeared the simplest especially compared to the peribulbar and retrobulbar methods. Moreover, patients receiving the topical technique generally require only light sedation.
So, when I personally was diagnosed with a cataract, I contacted a surgical colleague who was experienced with the topical technique. I was a little nervous on the day of surgery but once the operation was started and I received two doses of intravenous Versed 0.5 milligrams, the case proceeded quickly and totally without pain. That is, there was no pain except for the removal the drapes placed on my face. The adhesives on these drapes did hurt as the drapes were removed.
When I left the operating room area, my wife was incredulous since I was not wearing a patch and had no signs of having surgery! I felt fine but decided to take a nap that afternoon. I returned to work two days later and can only say that the improvement in my vision was miraculous and I have nothing but praise for every aspect of my care. I only wish there were other simple ways to reverse so completely the ravages of approaching old age. Way to go ophthalmologists!
Mitch Sosis, MD
Campus Eye Group
Hamilton Square, N.J.



