Retinoblastoma treatment has undergone significant evolution over the past 60 years, and has even come full circle. In 1954, researchers reported on the delivery of triethylene melamine via the carotid artery for the treatment of retinoblastoma.1 The next few decades saw a shift from primary enucleation, which remains a viable option for some tumors, to globe-salvaging therapies such as external beam radiation therapy. While EBRT was effective, significant side effects of secondary malignancies and facial disfigurement made it less than ideal. Systemic chemotherapy combined with laser consolidation has resulted in greater than 99 percent survival at specialized centers and many children maintaining vision (See Figures 1A & B). Concerns over systemic toxicities of chemotherapeutic agents in young infants and children have led to an investigation of focal, locally delivered therapies. In 2004, almost 50 years after the initial report on intracarotid delivery, super selective intra-arterial chemotherapy has stepped into the forefront in the treatment of retinoblastoma.
Just like in the 1950s, controversies abound with the adoption of new therapies and only time will reveal the full picture. This article discusses the controversies in retinoblastoma treatment.
Diagnosis
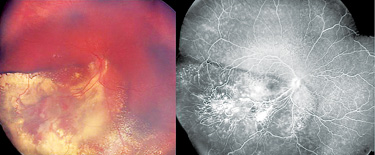
One of the most important initial factors in the treatment of retinoblastoma is making the correct diagnosis in a timely manner. The majority of children first come to our attention when either the parents or the pediatrician note leukocoria or strabismus. Once referred to an ophthalmologist, the practitioner relies on their clinical acumen, as biopsy in retinoblastoma is contraindicated. There are various simulating lesions, including Coats’ disease, familial exudative vitreoretinopathy (FEVR) and persistent fetal vasculature (PFV), as well as inflammatory conditions like toxocariasis. In addition, retinoblastoma can have a Coats’-like response,2,3 making the correct diagnosis even more challenging (See Figures 1A & B). Although retinoblastoma is primarily a clinical diagnosis, ophthalmologists have multiple adjuvant tools in their armamentarium.
| |||||||||
Treatment
Treatment of retinoblastoma yields success in greater than 99 percent at specialized centers, an amazing statistic in pediatric oncology. The key to favorable outcomes is timely diagnosis and a coordinated treatment plan, including a pediatrician, pediatric oncologist, ocular oncologist and possibly a neurointerventional surgeon. Retinoblastoma treatment must focus primarily on saving the child’s life, followed by the eye, and finally preserving vision. These sentiments echo the evolution of retinoblastoma treatment, with the shift from enucleation as primary management to globe-salvaging treatments such as EBRT and chemotherapy. However, as with any rare disease, retinoblastoma treatment does not have the luxury of large-scale clinical trials, thus evidence stems primarily from individual case series. An inherent problem is the lack of a standardized approach in treatment, especially as we forge ahead with new treatments.
As a result of significant side effects of EBRT, chemotherapy was investigated for globe-salvaging treatment. Current chemotherapy protocols include the use of vincristine, etoposide and carboplatin (VEC), with or without cyclosporine. An early controversy was sole use of systemic chemotherapy for retinoblastoma treatment. However, studies showed that chemotherapy-alone resulted in tumor control rates for Reese-Ellsworth (R-E) group I-IV of 51 to 65 percent.4,5 Conversely, when combined with focal laser consolidation, control rates increase to 62 to 100 percent.6,7 For advanced tumors (R-E group V), results are even more striking, with chemotherapy-alone showing tumor control in only 25 to 37 percent,4,5 while combined chemotherapy with focal laser resulted in control rates of 47 to 83 percent.6,8 These studies emphasize the importance of combined treatment to maximize tumor control in retinoblastoma. Additional controversies arise from the application of laser consolidation in the macula, notably extrafoveal versus foveal regions. Of note, using aggressive focal laser treatment with systemic chemotherapy resulted in 100 percent tumor control in R-E group I-IV, and 83 percent in R-E group V tumors. Despite aggressive laser treatment, 86 percent of children maintained 20/400 or better vision, while 56 percent maintained 20/80 or better vision.6
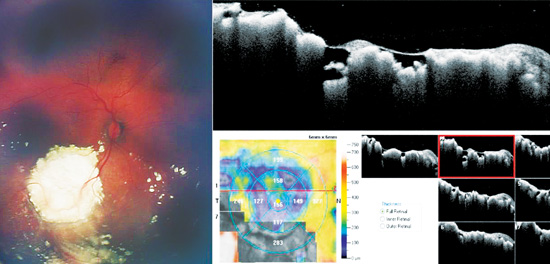
Systemic chemotherapy with focal laser consolidation has revolutionized the treatment of children with retinoblastoma, allowing for globe-salvage with many maintaining vision (See Figures 2A & B). However, systemic administration of toxic chemotherapeutics to infants and children is not ideal. Common side effects include anemia, thrombocytopenia and neutropenia, potentially requiring hospitalization and transfusions. Other significant, but controversial side effects include a risk of secondary malignancies and ototoxicity.
| |||||||||
Regarding secondary malignancies following chemotherapy, a study by Kiran Turaka, MD, and colleagues showed that the risk was 4 percent in 187 patients with germline mutations, while that risk was 0 percent for non-germline mutations.9 Malignancies observed at a mean of 11 years follow-up were osteosarcoma, rhabdomyosarcoma, melanoma, glioma and acute promyelocytic leukemia. There have also been reports of acute myelogenous leukemia (AML).10 An additional controversial toxicity is ototoxicity from platinum-based agents. Reports have highlighted the potential of this side-effect during long-term follow-up,11 with recent presentations from the Children’s Oncology Group indicating a low-risk of ototoxicity (Ann-Marie Leahey, MD, presentation at International Society of Ocular Oncology 2011). With the low risk of these complications, treatment should not be withheld for these concerns.
As a result of significant side effects with the use of toxic systemic chemotherapeutics, there has been a recent push towards locally-delivered therapies. Options for local delivery include intravitreal injection, a technique that is usually contraindicated secondary to the risk of extraocular extension, periocular injection and direct arterial delivery. There is much controversy regarding the use of intravitreal injection in an eye harboring an active retinoblastoma. Several investigators have recently reported the benefit of intravitreal melphalan as a salvage in advanced retinoblastoma. Arguments for intravitreal injection are that this route delivers the highest concentration to the vitreous. As most tumors fail treatment secondary to persistent vitreous seeding, intravitreal chemotherapy may provide adequate doses to effectively treat these tumor cell populations.
In separate reports at the 2011 International Society of Ocular Oncology meeting, Francis L. Munier, MD, et al., and Shigenobu Suzuki, MD, et al., reported on their experience with intravitreal chemotherapy. Both groups used varying concentrations of melphalan. The investigators emphasized a novel injection technique developed to minimize vitreous reflux and sterilize the needle track. Despite prior treatments with systemic chemotherapy and intra-arterial chemotherapy, for 23 patients, salvage rates were 87 percent, with no cases of extraocular extension (Munier, ISOO 2011).
In a larger series, 227 patients were treated with intravitreal melphalan, resulting in 58 percent globe-salvage, including 68 percent with active vitreous seeding. Of note, one patient (0.4 percent) had extraocular extension, and one patient (0.4 percent) had systemic metastasis where the association with the vitreous injection could not be eliminated (Suzuki, ISOO 2011). Although intravitreal chemotherapy shows promise in the salvage treatment of advanced retinoblastoma, the risk of extraocular extension, metastasis and death should not be taken lightly.
More recently, locally delivered treatments have revisited the short-lived arterial delivery first experimented in 1954. Selective intra-arterial chemotherapy for retinoblastoma was pioneered by a Japanese group in 2004.12 Using a ballooned catheter, the technique involved passing a catheter through the carotid artery past the ophthalmic artery ostium, followed by distal occlusion and administration of chemotherapeutics. This technique was later modified by David H. Abramson, MD, and colleagues by direct cannulation of the ophthalmic artery, or superselective intra-arterial chemotherapy.13 In 2008, preliminary evidence for this new technique showed globe-salvage in 78 percent (seven of nine) of patients with advanced retinoblastoma (R-E V) destined for enucleation. Importantly, there were no severe adverse effects. Ocular event-free survival at two years has been shown to be 81.7 percent as primary management, and 58.4 percent as salvage treatment.14 Other investigators have shown similar success with intra-arterial chemotherapy, with salvage rates of 76,15 and 50 percent,16 while the rate of tumor control for primary management was 67 percent.16 Of note, 100 percent of International Classification of Retinoblastoma group C and D eyes were successfully treated with intra-arterial chemotherapy; this decreased to 33 percent for advanced ICRB group E eyes.
|
Superselective intra-arterial chemotherapy remains in its infancy, utilized by a few specialized centers around the world. In its short career, IAC has not been without controversy. As with any new treatment, there is much to learn regarding this new technique. Although short-term results are favorable, we currently do not have long-term follow-up on tumor control, extraocular extension, metastasis and survival. In addition, toxicities of this treatment include vascular events and diffuse RPE pigmentary changes that may result in vision loss acutely or over time. Questions remain regarding treatment protocols, including medications used (melphalan, carboplatin), dosages of chemotherapeutic agents, timing of treatments, number of treatments, maximum number of catheterizations, and the use of concomitant focal laser consolidation.
Alternatively, there are several investigators who are opposed to the use of IAC in place of systemic chemotherapy, citing visually significant side effects and lack of long-term follow-up. There has also been some concern about a potential increase in trilateral retinoblastoma in hereditary cases treated with IAC as primary treatment. Again, as a result of the rarity of this disease, clinical trials designed to answer some of these questions are difficult. As with EBRT and systemic chemotherapy, only time will tell regarding the long-term safety and efficacy of this treatment. One thing is for sure, the controversies will continue. REVIEW
Dr. Houston is a resident in ophthalmology at Bascom Palmer Eye Institute in Miami, and associated with the Ocular Oncology Research Lab at BPEI. Dr. Murray is a professor of ophthalmology and director of Ocular Oncology at BPEI, Miller School of Medicine at the University of Miami. Correspondence can be addressed to Dr. Houston (
shouston@med.miami.edu) or Dr. Murray (
tmurray@med.miami.edu).
1. Reese AB, Hyman GA, Merriam GR, Jr., Forrest AW, Kligerman MM. Treatment of retinoblastoma by radiation and triethylenemelamine. AMA Arch Ophthalmol 1954;53:505-513.
2. Silva RA, Dubovy SR, Fernandes CE, Hess DJ, Murray TG. Retinoblastoma with Coats’ response. Ophthalmic Surg Lasers Imaging 42 Online:e139-143.
3. Shields CL, Uysal Y, Benevides R, Eagle RC, Jr., Malloy B, Shields JA. Retinoblastoma in an eye with features of Coats’ disease. J Pediatr Ophthalmol Strabismus 2006;43:313-315.
4. Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol 2003;21:2019-2025.
5. Gombos DS, Kelly A, Coen PG, Kingston JE, Hungerford JL. Retinoblastoma treated with primary chemotherapy alone: The significance of tumour size, location, and age. Br J Ophthalmol 2002;86:80-83.
6. Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG. Macular retinoblastoma: Evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology 2007;114:162-169.
7. Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology 2006;113:2276-2280.
8. Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol 2006;17:228-234.
9. Turaka K, Shields CL, Meadows AT, Leahey A. Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr Blood Cancer 2011 Aug 8. doi: 10.1002/pbc.23278. [Epub ahead of print]
10. Gombos DS, Hungerford J, Abramson DH, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: Is chemotherapy a factor? Ophthalmology 2007;114:1378-1383.
11. Jehanne M, Lumbroso-Le Rouic L, Savignoni A, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer 2009;52:637-643.
12. Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol 2004;9:69-73.
13. Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology 2008;115:1398-1404, 1404 e1391.
14. Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol 2011;129:732-737.
15. Peterson EC, Elhammady MS, Quintero-Wolfe S, Murray TG, Aziz-Sultan MA. Selective ophthalmic artery infusion of chemotherapy for advanced intraocular retinoblastoma: Initial experience with 17 tumors. J Neurosurg 2011;114:1603-1608.
16. Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma: Report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol 2011;129:1399-1406.
17. Eagle RC, Jr., Shields CL, Bianciotto C, Jabbour P, Shields JA. Histopathologic observations after intra-arterial chemotherapy for retinoblastoma. Arch Ophthalmol 2011;129:1416-1421.