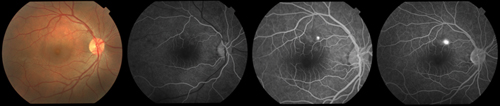
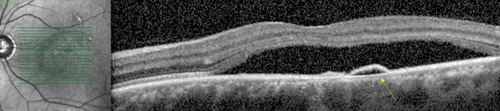
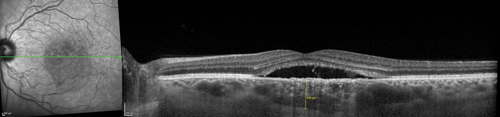
Multimodal imaging is useful in making the diagnosis of CSCR. Classically, fluorescein angiography demonstrates an expanding point of fluorescein leakage with late pooling into a serous detachment (See Figure 1). Multiple points of leakage can be seen in some patients.5,6 Indocyanine green angiography may show focal delays and hyperpermeability in the choroidal circulation in many patients with CSCR.7,8 Optical coherence tomography demonstrates subretinal fluid, often associated with a focal pigment epithelial detachment (See Figure 2).9 More recently, enhanced-depth imaging spectral domain OCT has shown increased subfoveal choroidal thickness in some patients with CSCR as compared to normal eyes (See Figure 3).10
The typical natural history of CSCR is complete spontaneous resolution of subretinal fluid with restoration of visual acuity by three months after onset of symptoms. However, up to 20 percent of patients may have persistent serous macular detachment and vision loss past six months, and may be left with some degree of subjective visual impairment such as micropsia or reduced color perception.11-13 If subretinal fluid has not resolved by three months, the patient is defined as having chronic CSCR, and treatment is often considered.
|
Treatment Options
There is no gold standard for treatment of persistent CSCR, and a number of therapies have been tried with varying success. Focal laser photocoagulation to pinpoint areas of leakage on FA was the first treatment shown to be of some benefit for CSCR.14 However, photocoagulation is destructive, can lead to symptomatic scotomas, and occasionally formation of secondary choroidal neovascularization. Therefore, this treatment is reserved for focal extrafoveal areas of dye leakage.
Photodynamic therapy more directly targets the choroidal circulation and may be used in patients with sub-foveal and/or multifocal points of leakage. PDT has been used for persistent CSCR with some success. However, it is not approved by the Food and Drug Administration for the treatment of CSCR and has a number of side effects, including photosensitivity to intravenous dye and choroidal hypoperfusion following treatment.15,16 Several recent studies have demonstrated the use of half-fluence and half-dose PDT in acute and chronic CSCR, with the goal of maintaining efficacy while minimizing risk.17-20
Anti-VEGF medications have a number of effects that are theoretically beneficial in CSCR, such as the upregulation of tight junctions between endothelial cells and reduction of vascular fenestrations.21-23 A study by Ji Won Lim, MD, and colleagues suggested that VEGF levels in the aqueous humor of patients with chronic CSCR may be elevated compared to normal eyes.24 Case studies and anecdotal reports of intravitreal anti-VEGF medications in patients with persistent or chronic CSCR have shown improvements in visual acuity, resolution of neurosensory detachments and decreased RPE leakage on FA.25-28 Prospective studies using anti-VEGF medications have shown inconsistent results.29,30 So far, however, the cumulative weight of evidence has failed to show sustained, clinically significant benefits.31 Controlled clinical trials are necessary to determine the tolerability and efficacy of anti-VEGF therapies in CSCR.
Several small studies have shown mixed results from a variety of systemic medications for CSCR, including carbonic anhydrase inhibitors (acetazolamide),32 adrenergic receptor antagonists (metoprolol, propranolol),33,34 and steroid hormone antagonists (ketoconazole, mifeprestone, finasteride, eplerenone).35-38
Eplerenone, a selective aldosterone-receptor antagonist and potassium-sparing diuretic that was originally approved in 2002 by the FDA for treatment of hypertension, was recently shown in a small series of patients with chronic CSCR to improve visual acuity and significantly decrease central macular thickness.39 The medication is generally well-tolerated but drug interactions must be ruled out prior to initiation and serum potassium and blood pressure must be monitored during treatment. Larger, prospective, placebo-controlled studies are under way to further investigate the efficacy of this treatment option.40 Currently, pharmacologic treatments for CSCR remain investigational and are not considered standard of care. If medically appropriate, systemic corticosteroids should be discontinued in patients with active CSCR. A sleep study may be considered in patients with suspected obstructive sleep apnea.41
Central serous chorioretinopathy is a disease of working-aged patients, many of whom have occupations that demand high levels of visual acuity. Characteristic angiographic and OCT findings are helpful in confirming the diagnosis. While the majority of patients will return to baseline with observation, a subset of patients may be considered for intervention. No therapeutic options are approved by the FDA, but local modalities, both pharmacologic and photic, and systemic medical treatments are under ongoing investigation and may hold promise for future patients diagnosed with CSCR. REVIEW
Dr. Pitcher is a second year fellow in vitreoretinal surgery at Wills Eye Hospital and a clinical instructor of ophthalmology at Thomas Jefferson University in Philadelphia. He can be reached at johndpitcher@gmail.com.
Dr. Hsu is an assistant professor of ophthalmology on the Retina Service of Wills Eye Hospital and practices at Mid Atlantic Retina. He can be reached at jhsu@midatlanticretina.com.
1. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmologica 2008;86:126-45.
2. Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology 2008;115:169-73.
3. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967;63:1-139.
4. Ross A, Ross AH, Mohamed Q. Review and Update of Central Serous Chorioretinopathy. Curr Opin Ophthalmol 2011;22:166-73.
5. Yamada K, Hayasaka S, Setogawa T. Fluorescein-angiographic patterns in patients with central serous chorioretinopathy at the initial visit. Ophthalmologica 1992;205:69-76.
6. Spitznas M, Huke J. Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefe’s Arch Clin Exp Ophthalmol 1987;225:437-40.
7. Kitaya N, Nagaoka T, Hikichi T, et al. Features of abnormal choroidal circulation in central serous chorioretinopathy. Br J Ophthalmol 2003;87:709-12.
8. Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010;117:1792-9.
9.Shinojima A, Hirose T, Mori R, et al. Morphologic findings in acute CSC using spectral domain OCT with simultaneous angiography. Retina 2010;30:193-202.
10. Imamura Y, Fujiwara T, Margolis RON, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 2009; 29:1469-73.
11. Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol 1984;68:815-20.
12. Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmologica Scandinavica 2001; 79:417–21.
13. Singer M, et al. Non-steroidal anti-inflammatory topical therapy speeds recovery in central serous chorioretinopathy. AAO 2013 meeting, New Orleans. Unpublished data.
14. Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1979;63:674-77.
15. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: A pilot study. Retina 2003;23:288-298.
16. Piccolino FC, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003;23:752-763.
17. Smretschnig E, Ansari-Shahrezaei S, Hagen S, Glittenberg C, Krebs I, Binder S. Half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Retina 2013:33:316-23.
18. Chan W, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy. Ophthalmology 2008;115:1756-1765.
19. Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina 2011;31:119-126.
20. Bae SH, Heo J, Kim C. Low-Fluence Photodynamic Therapy versus Ranibizumab for Chronic Central Serous Chorioretinopathy: One-Year Results of a Randomized Trial. Ophthalmol 2013 Nov 20. S0161-6420(13)00844-0. doi: 10.1016/j.ophtha.2013.09.024. [Epub ahead of print]
21. Witkin AJ, Brown GC. Update on nonsurgical therapy for diabetic macular edema. Curr Opin Ophthalmol 2011;22:185-9.
22. Chiang A, Regillo CD. Preferred therapies for neovascular age-related macular degeneration. Curr Opin Ophthalmol 2011;22:199-204.
23. London NJ, Brown G. Update and review of retinal vein occlusion. Curr Opin Ophthalmol 2011;22:159-65.
24. Lim JW, Kim MU, Shin M-C. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina 2010;30:1465-1471.
25. Kim H-S, Lee JH. The short-term effect of intravitreal bevacizumab for treatment of central serous chorioretinopathy. J Korean Ophthalmol Soc 2010;51:860-864.
26. Torres-Soriano M, Garcı´a-Aguirre G, Kon-Jara V, et al. A pilot study of intravitrealbevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefe’s Arch Clin Exp Ophthalmol 2008;46:1235-1239.
27. Seong HK, Bae JH, Kim ES, et al. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: Short-term effect. Ophthalmologica 2009;223:343-347.
28. Lim JW, Ryu SJ, Shin M-C. The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol 2010;24:155-158.
29. Bae SH, Heo JW, Kim C, et al. A randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol 2011;152:784-92.
30. Artunay O, Yuzbasioglu E, Rasier R, et al. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: A prospective, controlled clinical study. Curr Eye Res 2010;35:91-98.
31. Chung Y-R, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: Meta-analysis and review. Eye 2013;27:1339-46.
32. Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology 2002;109:1723-5.
33. Avci R, Deutman AF. Treatment of central serous choroidopathy with the beta receptor blocker metoprolol (preliminary results). Klin Monbl Augenheilkd 1993;202:199-205.
34. Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: An evaluation by serial OCT. J Ocular Pharmacol Therapeut 2006;22:145-9.
35. Golshahi A, Klingmuller D, Holz FG, Eter N. Ketoconazole in the treatment of central serous chorioretinopathy: A pilot study. Acta Ophthalmologica 2010;88:576-81.
36. Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina 2011;31:1928-36.
37. Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB. Finasteride for chronic central serous chorioretinopathy. Retina 2011;31:766-71.
38. Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest 2012;122(7):2672-9.
39. Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: A pilot study. Retina 2013;33:2096-102.
40. http://clinicaltrials.gov/ct2/show/NCT01990677
41. Yavas GF1, Küsbeci T, Kasikci M, Günay E, Dogan M, Unlü M, Inan UÜ. Obstructive sleep apnea in patients with central serous chorioretinopathy. Curr Eye Res 2014 39(1):88-92.