Review of Cornea & External Disease In this Issue... Artificial Intelligence for the Cornea Specialist by Christine Yue Leonard DSO and Cultured Endothelial Cell Transplants: A Review by Thomas John, MD, and Anny M.S. Cheng, MD Premium IOLs in Patients with Corneal Conditions by Asim Piracha, MD Diagnosis & Management of Blepharitis by Charles Bouchard, MD, MA |
Uveitis can be a challenging condition to manage, with a dizzying array of treatment options. These options have varying degrees of efficacy, selection criteria for use, as well as different side effects that also must be taken into account when you’re choosing a treatment path. Here, I’ll review our current options for quelling uveitis and avoiding medication side effects as best we can.
Diagnosing Uveitis
Uveitis is traditionally defined as the inflammation of the uveal structures, which include the iris, ciliary body and/or choroid.1 The site of involvement, such as anterior, intermediate, posterior or panuveitis, categorizes the disease.2 Untreated, uveitis can cause visual decline through many mechanisms, including, but not limited to, macular edema, optic nerve edema and cataract.3 All new uveitis patients, and follow-up patients not responding as expected, should be dilated to best categorize their site of involvement, as this can formulate a more precise diagnosis (Figure 1).
The diagnosis and management of uveitis can be tricky for multiple reasons: The disease has variable presentations, and patients sometimes provide an incomplete history or are difficult to examine (particularly children). In addition, uveitis is fraught with visually threatening complications and/or systemic manifestations. Despite all this, with the correct diagnostic approach, a concise differential can be reached, and thankfully, multiple treatments exist to quell inflammation. When choosing a therapeutic agent, it’s essential to balance the ability of these drugs to induce disease remission against their potential side effects and toxicities.
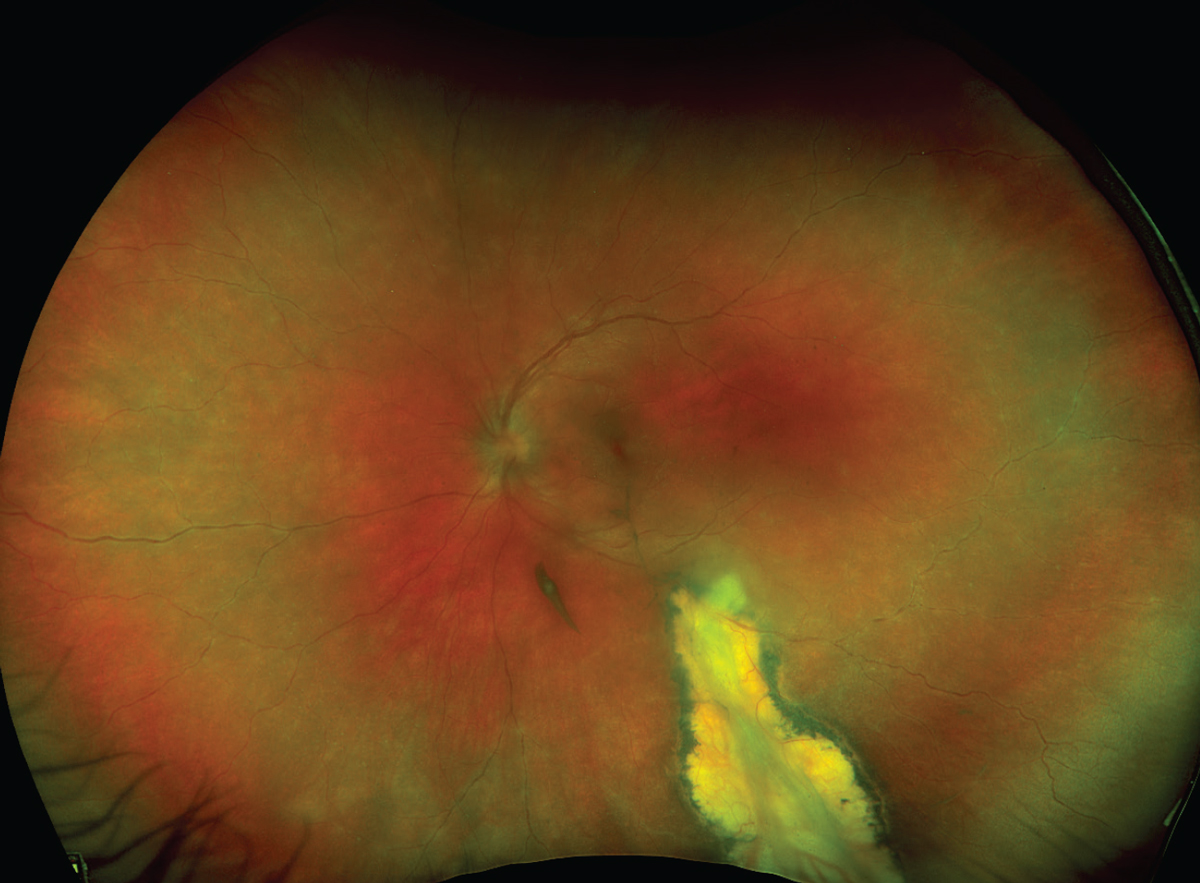 |
| Figure 1. A 17-year-old female sent in for evaluation for presumed anterior uveitis. Dilation showed intermediate and posterior involvement with a large chorioretinal scar consistent with toxoplasmosis. There’s an area of re-activation at the posterior edge of the lesion as well as vascular sheathing. |
This review will focus on intermediate, posterior and panuveitis treatment, which have a combined prevalence of 23 per 100,000 adults, and are even less common in children.4 When approaching treatment, it’s important to understand the underlying cause, as this will direct your treatment algorithm. The manifestations of uveitis are either primary, where the eye is the only site of known involvement, or secondary to a systemic immune or infectious condition. In terms of systemic conditions, infectious disease is much more common in developing countries (with rates as high as 50 percent of all cases), while non-infectious causes are the most common causes of uveitis in more developed areas.4 When working up a patient with uveitis, the three most common diagnoses I consider and/or want to rule out for treatment considerations are sarcoidosis, syphilis and tuberculosis, but my differential is tailored extensively after taking into consideration the patient’s medical history and exposures. In any case of suspected infection, perform an aqueous or vitreous tap before administering steroids, especially before administering local or periocular deposits.
As a side note, in children, disease can be especially difficult to recognize and treat, and in certain cases an examination under anesthesia can be necessary if an outpatient examination is low-yield. Recognizing uveitis is difficult in these patients, as they can be asymptomatic or have more chronic disease, and/or they may not be able to verbalize their issues at all.5 Moreover, visual complications result in amblyopia in at-risk age groups, with long-lasting social and economic ramifications. In these situations, I recommend consulting with a uveitis specialist for evaluation and management early on. I also advise a careful examination in cases of trauma-related uveitis, particularly in children, to rule out cases of globe rupture or retained foreign body (Figure 2).
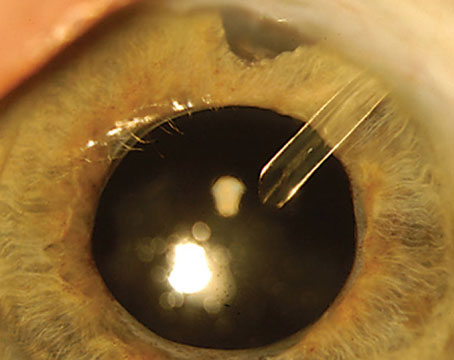
 |
| Figure 2. A 23-year-old male sent for assessment for traumatic iritis with a difficult examination at the slit lamp. Careful ocular evaluation revealed a full thickness corneal laceration, iris plugging of the wound, iris peaking and evidence of early endophthalmitis; orbital imaging showed a retained metallic foreign body. |
Treatment
Treatment aims at obtaining quiescence of the disease, either by treating the infectious agent or treating the immune condition. Remember, in cases of suspected immune disease that don’t improve or even worsen with steroids or immunosuppression, consider the possibility of infection or malignancy.
Following are the treatments at your disposal:
Corticosteroids
The initial therapy for uveitis is often corticosteroids, which can act quickly to quiet inflammation. Briefly, their mechanism of action relies on their ability to bind to receptors within cells involved in the inflammatory cascade, ultimately leading to the downregulation of pro-inflammatory molecules and cytokines.3 Given their widespread effect at dampening inflammation, they’re instrumental, but I remind patients that they’re often not an ideal long-term option.
As you know, steroids come in various levels of intensity. In new patients, or patients for whom their diagnosis isn’t clear, I prefer to stick to methods that I can quickly stop or change. In these patients, when the inflammation is mild, I often stick with topical therapies while completing their work-up, in case an infectious cause is found. However, in cases of advanced intermediate, posterior, or panuveitis, I’ll dispense a small amount of oral steroids, emphasizing to the patient the importance of adherence to the drug regimen, and I’ll order lab work so I can monitor their response to the treatment and complete their work-up. I don’t administer local steroids (like posterior sub-Tenon’s Kenalog or intravitreal steroids) until I’m sure I’ve ruled out infection. In cases of recurrent or recalcitrant inflammation, I start the discussion of steroid-sparing therapy early.3
Topical steroids, such as prednisolone acetate 1% or difluprednate 0.05%, have good effects on anterior uveitis, but I’ll also use them in mild cases of more posterior-involving uveitis. I upgrade to difluprednate if there’s more posterior inflammation or macular edema, as it’s been shown to have higher rates of vitreous penetration than prednisolone, keeping in mind that this is a higher strength topical steroid and carefully monitoring for steroid issues, such as cataract and glaucoma.6,7 I have a low threshold for increasing treatment to oral or local therapies once infection is ruled out, as undertreatment of uveitis is linked to worse visual outcomes. If the patient has anterior chamber cell or is forming posterior syenchiae, I’ll also start dilating drops to prevent or break up the synechiae, and keep track of the formation of synechiae in case they progress to iris bombe and require a laser peripheral iridotomy. Topical therapies are also useful in testing a patient’s steroid response, which can be kept in mind when considering local steroid therapies. Punctal occlusion can help reduce any systemic exposure of the drugs.
In patients with more extensive uveitis, oral prednisone is most commonly used, with the dose ranging between 1 to 1.5 milligrams/kilogram, but, given the side effects, I rarely dose patients above 80 mg even if their weight would dictate a higher amount. I move towards oral therapy quickly when patients have issues that also require quicker intervention, especially when there’s foveal-adjacent pathology or they’re monocular. When starting oral steroids, I keep in mind my endpoints for steroid treatment, so when they’re reached I can begin tapering the medication. The rationale for this is twofold: a tapering schedule should be followed after two weeks of high-dose oral steroids instead of abruptly stopping them, as this has been linked to adrenal insufficiency. Additionally, I try to get patients off of oral steroids within a reasonable time frame, since they have a multitude of side effects, including decreased bone density; peptic ulcers; Cushing syndrome; blood sugar and blood pressure deregulation; weight gain; immunosuppression; and mood deregulation.8 The literature has shown that a maintenance dose of 7.5 mg daily or less has the least systemic side effects and I work towards this with patients, but would ideally like them off steroids in the long term.3 Oral steroids are generally considered safe in pregnancy, but there have been reports of steroid use being linked to cleft palate in the fetus, particularly with use during the first trimester, though this is debated.9 In children, I’m especially cognizant of the amount and length of steroid use as this can lead to osteonecrosis.
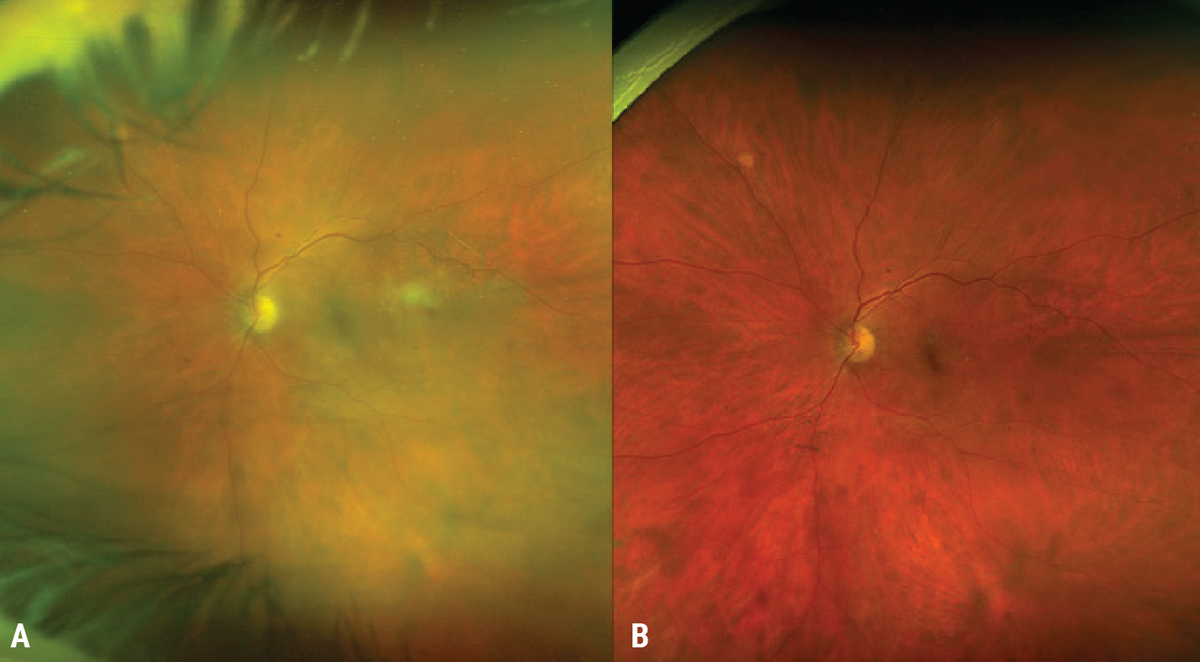 |
| Figure 3. A 59-year-old female presenting with bilateral intermediate uveitis (A) that required 60 mg of oral steroids with an appropriate taper after infectious etiologies were ruled out (B). |
In rare cases, I’ll initiate intravenous corticosteroids with careful serial ocular examinations and after I’ve ruled out infection, especially in patients with severe inflammation, foveal-threatening lesions, optic nerve involvement or monocular status. An IV pulse of methylprednisone (1 gram daily, for three days), with plans to transition to an oral regimen is my usual approach.
A myriad of options exist for local therapy, including periocular depots, intravitreal injections and implants, surgical implants and suprachoroidal administration. The advantages of local therapy include avoiding systemic side effects as well as a more direct administration of therapy. However, some of the general disadvantages of local therapy include cataract, steroid induced glaucoma and difficulty in removing the steroid if necessary. As stated prior, I always rule out infection before administering local steroid.
Periocular therapy, usually consisting of triamcinolone acetonide 40 mg/ml can be injected into the sub-conjunctival space (usually in cases of anterior scleritis), and in cases of more posterior involvement, trans-septally or in the sub-Tenon’s space.3 Typically, a 27-gauge needle is used to administer 40 mg/ml of triamcinolone acetonide; if performing a trans-septal injection an inferior approach is usually taken, while a sub-Tenon’s injection can either be superotemporal or inferior. When engaging the trans-septal or sub-Tenon’s space, I aim posterior to the equator and move the needle laterally and medially to make sure I haven’t engaged the globe itself, as this can lead to retinal tears, detachment, and administration of steroid intra-ocularly. Both injections are associated with cosmetic complications: the inferior administration of trans-septal steroid can cause fat prolapse, and superior sub-Tenon’s injection can cause injury to the
levator palpebrae, resulting in ptosis.
Intravitreal injections provide another avenue of steroid implementation, particularly in patients who can’t tolerate steroids or other immunosuppressive therapies, those who fail periocular administration, don’t respond enough to oral steroids, or have immediate, vision threatening pathology.10 Whenever I administer an intravitreal steroid, I prefer to use of 2 to 4 mg of preservative-free triamcinolone acetonide. The disadvantage of this medication, though, is its relatively short duration, (usually three months, but it can last slightly longer), requiring frequent re-administrations.11 In certain patients that demonstrate a favorable response to intravitreal triamcinolone, I follow that bolus with an intravitreal implant to gain longer lasting effects.
Two types of intravitreal implants exist: The 0.7 dexamethasone implant (Ozurdex, Allergan) and the flucinolone aceonide 0.18 mg implant (Yutiq, EyePoint Pharmaceuticals).
The Ozurdex implant is a completely biodegradable dexamethasone implant with a lactic-acid glycolic matrix; this matrix is what leads to corneal degradation in the cases where the implant migrates into the anterior chamber. The injector is 22 gauge, and I often used a beveled incision when delivering the drug, much like inserting a trocar for vitrectomy. The medication may last for up to six months, but often peaks at two months, with a small residual effect lasting for up to three to four months after injection.12
The Yutiq implant is a non-biodegradable implant composed of a polyamide cylinder with internal steroid that delivers a slow, low-rate release of steroid over three years.13 Compared to Ozurdex, this theoretically could provide a patient with fewer injections and long-lasting control, but in patients with profound inflammation, Yutiq may not provide enough coverage. I also find the Yutiq implant useful in cases of post-cataract driven inflammation and macular edema, as the inflammation in these cases is usually mild. The disadvantages of Yutiq include a possibility of anterior chamber migration, and the accumulation of multiple Yutiq devices with repeated injections.
When debating the type of local treatment, I often consider the results of the POINT study, which compared the use of Ozurdex versus intravitreal triamcinolone versus sub-Tenon’s triamcinolone; all groups showed a significant improvement in visual acuity and central retinal thickness; however, the intravitreal groups showed a significant difference in the reduction of central retinal thickness and improvement in visual acuity compared to the sub-Tenon’s group. The intravitreal patients had a higher rate of intraocular pressure rise, and because of this, in patients with a history of steroid response, I tend to stay with more periocular administrations if local steroid therapy is necessary.
The use of the suprachoroidal space has become more popular in drug development, and recently gave rise to the approval of Xipere (triamcinolone acetonide injectable suspension, Bausch + Lomb/Clearside Biomedical) 4 mg/0.1 ml.14 Xipere is administered into the suprachoroidal space with a micro-needle that comes in two sizes. This is advantageous in patients with mostly posterior pathology, and theoretically has a lesser risk of developing cataract or steroid response pressure rises in patients due to the posterior depot of the medication.14 Additionally, I consider this medication in patients without an intact lens barrier, or those who are aphakic, as I would be concerned about drug migration with an intravitreal administration. The disadvantages of this medication are that there can potentially be inadvertent entry into the globe resulting in retinal detachment or tears, as well as choroidal pathology.14
A surgical implant also exists for the treatment of intermediate, posterior and panuveitis; a 0.59 mg flucinolone implant (Retisert, Bausch + Lomb), which is sutured to the sclera and lasts for up to three years, if not longer.15 The Multicenter Uveitis Steroid Treatment Trial showed increased control with the Retisert implant compared to systemic therapy in uveitic eyes (88 percent vs. 71 percent) and equal visual acuity at 24 months. At seven years, however, the visual acuity was better in the systemically treated group.16 The disadvantages of this therapy include a large bolus of steroid that increases the risk of cataract, steroid-related pressure increases, intraocular bleeding, and hypotony with poor wound closure, and results in the loss of scleral and conjunctival real estate that would have been useful for other ocular procedures down the line, especially if multiple implants are needed.
Steroid-sparing Therapies
In patients whose inflammation is recurrent or whose steroids can’t successfully be tapered down to appropriate maintenance dosing, or who are experiencing complications with local or systemic steroid therapy, steroid-sparing treatments are an excellent option. Treatment options in this class include antimetabolites, biologics and alkylating agents.
• Antimetabolites. Methotrexate, a folic acid analog, is often used in both adults and children given its well-researched safety and efficacy profile.17 It can be administered subcutaneously or orally, and dosages typically range from 10 to 25 mg. I prescribe this with a daily folic acid to help curb some of the side effects of the medication, which include oral ulcers, gastrointestinal discomfort and hair loss. Serious clinical and lab manifestations include interstitial pneumonitis, alterations in liver function tests and bone marrow suppression.17 In order to monitor patients, baseline labs should be obtained, and routine lab work should be done every three months.
Azathioprine and mycophenolate mofetil are two other antimetabolites, both of which inhibit purine synthesis, in turn blocking the maturation of lymphocytes. Azathioprine is dosed at 1-3 mg/kg/day, while mycophenolate is administered at a maximum of 1,500 mg twice a day. The most common side effects include gastrointestinal distress, but serious complications can also arise, including bone marrow suppression. Thus, like methotrexate, baseline labs as well as lab work every three months should be done to monitor for these. Of these anti-metabolites, only azathioprine is safe in pregnancy. Anti-metabolites should generally not be combined with another anti-metabolite, however they can be used in combination with other forms of steroid sparing drugs, such as anti-TNF-α medications.
• Biologics. TNF-α inhibitors are mainstays of the biologic class of drugs. These medications target TNF-α; a potent cytokine in the inflammatory pathway, and are generally well-tolerated in both adults and children. In cases where a patient can’t tolerate anti-metabolites, or needs to be transitioned quickly to a steroid-sparing agent, I’ll often consider an anti-TNF-α medication. The biologic adalimumab is a fully humanized anti-TNF- α monoclonal antibody, which has been shown to be effective in intermediate, posterior and panuveitis, as well as in children with JIA.18-20 Dosage of adalimumab is typically 40 mg, administered subcutaneously, every other week, but in certain patients, it can be used on a weekly basis. Another biologic agent is infliximab, a chimeric monoclonal antibody with proven results against posterior uveitis in both children and adults, often dosed between 5 mg/kg and 10 mg/kg via infusion.
Other agents in this class include etanercept, golimumab, and certolizumab; with the last two having modest amounts of literature supporting their use in uveitis. Conflicting evidence exists regarding anti TNF- α medications in pregnancy, but they’re generally considered safe in early phases of gestation.
There’s also rituximab, a monoclonal antibody focused against CD20; and tocilizumab, a recombinant monoclonal antibody targeting IL-6, intravenous immunoglobulin or interferon. Tocilizumab has proven particularly promising in reducing vascular leakage and macular edema.
Alkylating agents
Rarely, alkylating agents, such as cyclophosphamide and chlorambucil, may be used for severe, refractory cases of uveitis, but their toxicity profile is large and robust. When initiating treatment in women of childbearing age, always consult with rheumatology and OB/GYN before starting immunosuppression, and confirm the lack of pregnancy.
In conclusion, a myriad of therapeutic options exists for intermediate, posterior and panuveitis patients. Typically, the algorithm consists of ruling out infectious etiologies, starting with corticosteroids and—if unable to taper down the steroid or obtain adequate control—the addition of steroid-sparing therapies.
Dr. Arepalli is an assistant professor in the Vitreoretinal and Uveitis Service at Emory University.
1. Nussenblatt RB. The natural history of uveitis. International ophthalmology 1990;14:303-8.
2. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509-16.
3. Valdes LM, Sobrin L. Uveitis therapy: The corticosteroid options. Drugs 2020;80:765-73.
4. Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmology 2016;134:1237-45.
5. BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. The British Journal of Ophthalmology 2005;89:444-8.
6. Sheppard JD, Foster CS, Toyos MM, et al. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: Pooled efficacy analysis of two phase 3 studies. Ocular Immunology and Inflammation 2019;27:484-96.
7. Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol 2012;153:932-8.
8. Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. StatPearls. Treasure Island (FL): StatPearls Publishing, 2022. [online article].
9. Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheumatic Disease Clinics of North America 2017;43:489-502.
10. Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology 2005;112:1916:e1-7.
11. Ganapathy PS, Lowder CY, Arepalli S, et al. Treatment duration and side effect profile of long-term use of intravitreal preservative-free triamcinolone acetonide in uveitis. Am J Ophthalmol 2018;194:63-71.
12. Lam WC, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: The CHROME study. Clinical ophthalmology 2015;9:1255-68.
13. Jaffe GJ, Lin P, Keenan RT, Ashton P, Skalak C, Stinnett SS. Injectable fluocinolone acetonide long-acting implant for noninfectious intermediate uveitis, posterior uveitis, and panuveitis: Two-year results. Ophthalmology 2016;123:1940-8.
14. Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal cls-ta for macular edema secondary to noninfectious uveitis: Phase 3 randomized trial. Ophthalmology 2020;127:948-55.
15. Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology 2000;107:2024-33.
16. Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: The multicenter uveitis steroid treatment trial. Ophthalmology 2011;118:1916-26.
17. Chan NS, Choi J, Cheung CMG. Pediatric uveitis. Asia-Pacific Journal of Ophthalmology 2018;7:192-9.
18. Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med 2016;375:932-43.
19. Suhler EB, Adan A, Brezin AP, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: VISUAL III. Ophthalmology 2018;125:1075-87.
20. Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med 2017;376:1637-46.