A suprachoroidal hemorrhage is one of the most dreaded surgical complications an anterior segment surgeon can face. Although more frequently encountered when large-incision intracapsular and extracapsular cataract extractions were commonplace, this potentially catastrophic event can occur even during routine, small-incision phacoemulsification.
Here, we’ll review the risk factors and pathophysiology of this potentially devastating complication and offer advice on how to recognize and respond to it. Included are strategies for following up in the hours and days after a suprachoroidal hemorrhage.
What the Evidence Tells Us
Most studies examining SCH during cataract surgery don’t distinguish between phacoemulsification and ECCE. Estimates of incidence range from 0.04 to 0.26 percent.1,2,3,4 A Swedish study reported a 0.03 percent incidence of SCH among a cohort of more than 23,000 eyes undergoing phacoemulsification surgery, which is about three per 10,000 cases.5 These numbers may be falsely reassuring—although the risk of SCH may be very low for any case, most cataract surgeons will have to contend with a case at some point.
Risk Stratification
Risk factors for intraoperative SCH are categorized as systemic, ocular or intraoperative. (See Table 1, page 16.) Although most of these risk factors aren’t modifiable, you can control two notable exceptions: those associated with systolic blood pressure and heart rate. Both are important perioperative considerations for optimization, especially in an already high-risk patient.
 |
The common thread connecting all risk factors listed table 1 is that they predispose a patient to rupture of a ciliochoroidal vessel, either because of structural weakness of the vessel or because of an increasing pressure gradient between the choroidal vasculature and the vitreous cavity. Once a vessel ruptures, rapid accumulation of blood occurs in the suprachoroidal space. Arterial and venous suprachoroidal bleeds can occur. Bleeding from an arterial hemorrhage accumulates much more rapidly, causing an elevation of the overlying and adjacent retina and choroid, which can then stretch, leading to the rupture of additional ciliary vessels. If all wounds are secured, IOP will rise and the accumulating hemorrhage will self-tamponade. If wounds remain open, extrusion of intraocular contents can occur.
 |
Intraoperative Warning Signs
An SCH can occur at any time during surgery. However, one study found the highest incidence (around 35 percent) during irrigation/aspiration after nucleus removal. The telltale sign of a developing hemorrhage is a darkening or distortion of the red reflex, often moving from one end of the eye to the other, although it can also remain in one quadrant. The anterior chamber can shallow, sometimes abruptly, particularly if the eye is hypotonous.
 |
IOP can spike, leading to a “rock-hard” globe and acute pain, especially if surgery is performed under topical anesthesia. Patients often complain of pain from concomitant stretching of the supraciliary nerves. They may involuntarily bear down, adding to the posterior pressure and pain. Intraocular viscoelastic may expulse, and the iris and IOL may prolapse. If the patient develops a capsular or zonular breach, look for vitreous presentation.
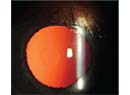
The hemorrhage may appear as a brownish, dome-shaped elevation on indirect ophthalmoscopy. But don’t stop to visualize it until you have secured the globe, as described below.
 |
Responding to an SCH
At the first indication of a possible SCH, your most critical and time-sensitive priority should be to withdraw your surgical instruments and achieve a watertight closure of all surgical wounds. (Table 2 below outlines basic guidelines for responding when you suspect this emergency.) If a wound remains open in the presence of an expansile SCH, you risk the extrusion of intraocular contents. Immediately suturing the wounds is particularly important during planned ECCE or conversion to ECCE from phacoemulsification, due to the larger incisions, which may not be self-sealing.
Although we’re conditioned as surgeons to avoid leaving nuclear material in the eye, you must resist the urge to finish the cataract procedure. Trying to remove remaining lens material increases the risk of a progressive SCH and subsequent loss of intraocular contents. When confronted by active suprachoroidal bleeding, particularly arterial bleeding, you won’t be able to infuse fluid fast enough to counteract the expansile forces of the hemorrhage. Arterial blood pressure is higher than infusion pressure, meaning fluid will be displaced back up the phaco tubing as the hemorrhage progresses. Even brief hypotony must be avoided. Any remaining steps, such as an anterior vitrectomy, are also better left for when the hemorrhage has stabilized or resolved. An attempted vitrectomy by an anterior segment surgeon risks iatrogenic damage to the retina and may allow extension of the hemorrhage.
Pre-placed sutures, recommended in high-risk patients, can be rapidly tied in response to a developing SCH, functioning as an important safeguard. As we know, wounds in standard phaco surgery are often self-sealing, as long as instruments have been removed from each (well-constructed) wound. But you should still consider safety sutures when a larger-than-normal incision is required, particularly in patients with risk factors for SCH.
Once you’ve sealed the surgical incisions, the globe will pressurize, counteracting the expansile force exerted by progressive suprachoroidal bleeding, and the hemorrhage will be tamponaded.
Some surgeons will intraoperatively drain an SCH via scleral cutdown. But this is typically not necessary unless you’re unable to close the surgical wounds and pressurize the globe. Furthermore, the skills to safely and rapidly create scleral windows aren’t commonly held among anterior segment surgeons. If you are unable to close the surgical wounds and pressurize the globe, seek intraoperative help from a vitreoretinal colleague, if available.
Follow-up and After-care
After intraoperative tamponade of the eye, monitor your patient closely to ensure stabilization of the hemorrhage and a return to a normotensive state with a formed anterior chamber. Postop topical steroids and cylcoplegia are important for controlling pain and inflammation. Treatment of IOP may also be needed. Follow the size and location of the hemorrhage with indirect ophthalmoscopy and/or B-scan ultrasound if a good view of the posterior segment is not possible.
 |
| Image courtesy of Christopher Riemann, MD |
If you had to abort cataract surgery to secure the globe when the SCH developed, you can complete any remaining steps after the hemorrhage has stabilized and the IOP and anterior chamber depth have been normalized. In some cases, you may be able to safely finish the surgery within hours to days of the original procedure. Optimal timing will vary from case to case. Consider early referral to a vitreoretinal colleague for help in evaluation and management. Drainage is typically only considered for “kissing choroidals” (appositional SCHs) and is generally performed a week to 10 days after onset of the SCH, when the clotted hemorrhage begins to liquefy. The hemorrhage may be drained intraoperatively or be allowed to resorb spontaneously. In either case, retinal detachment is common because vitreous gel is frequently incarcerated in anterior segment wounds as the suprachoroidal fluids resolve.1 Spontaneous resolution may take weeks or months.
Parting Advice
Even though SCH is rare, most cataract surgeons will encounter at least one during their careers. Above all, remember that rapid recognition of the problem and prompt watertight closure of all surgical wounds can prevent a catastrophic outcome. REVIEW
 |
Kavitha R. Sivaraman, MD is a partner at the Cincinnati Eye Cincinnati Eye Institute and assistant professor of ophthalmology-affiliated at the University of Cincinnati. She reports the following financial relationship: Consultant, W.L. Gore.
1. Ling R, Cole M, James C, et al. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004 88:4:478-80.
2. Desai P, Minassian DC, Reidy A. National cataract surgery survey 1997-8: A report of the results of the clinical outcomes. Br J Ophthalmol 1999;83:12:1336-40.
3. Stein JD1, Grossman DS, Mundy KM, et al. Severe adverse events after cataract surgery among Medicare beneficiaries. Ophthalmology 2011;118:9:1716-23.
4. Stein JD. Serious adverse events after cataract surgery Curr Opin Ophthalmol. 2012;23:3:219-25.
5. Eriksson A, Koranyi G, Seregard S, Philipson B. Risk of acute suprachoroidal hemorrhage with phacoemulsification. J Cataract Refract Surg. 1998;24:6:793-800.
6. Speaker MG, Guerriero PN, Met JA, et al. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology 1991;98:2:202-9; discussion 210.
7. Ling R, Kamalarajah S, Cole M, et al. Suprachoroidal haemorrhage complicating cataract surgery in the UK: Acase control study of risk factors. Br J Ophthalmol.2004;88:4:474-7.