Point: Use of MicroPulse TLT Should Be Expanded
Data suggests this technology could benefit many relatively healthy eyes, not just those with advanced glaucoma.
By: Sandra F. Sieminski, MD
MicroPulse TLT is a great tool to have in your armamentarium for treating glaucoma. Prior to the advent of MicroPulse, if your patient had advanced glaucoma, the main treatment options were trabeculectomy or a tube, surgeries that are fraught with postop complications and often require frequent postop care. (The advent of minimally invasive glaucoma surgeries, or MIGS, has provided another option that involves less follow-up, but MIGS procedures are largely utilized in early glaucoma.)
The current version of MicroPulse is a great tool for several reasons: it’s effective; it’s easy to do; it requires minimal procedural training; it’s portable; and it requires much less postop care than a trab or tube. The complication rate is very low and postop pain is generally negligible. It’s a quick procedure that’s easy to fit into your schedule. It only takes a couple of minutes, and there’s no prepping or draping. You don’t need an OR or even an operating microscope to perform the procedure. (In fact, the portability of this technology has made it widely used in many other countries; I often get questions about it from colleagues in Africa and the Middle East.)
Currently, most surgeons reserve MicroPulse for the treatment of eyes with advanced glaucoma—eyes in danger of losing most or all vision—as well as eyes that are already blind. Here, I’ll discuss the MicroPulse technology and how the procedure is currently performed, and share some of the data that supports the idea that it should be an option for treating much-less-damaged eyes as well.
The Technology
The current version of this treatment is referred to as micropulse transscleral laser therapy, or MP-TLT. The earlier form of this laser, called diode transscleral cyclophoto-
coagulation (TSCPC) was designed to partially destroy—or at least decrease the function of—the ciliary body, using thermal energy. This effectively lowered the rate of aqueous production. It was historically reserved for end-stage or blind eyes, because the destruction caused widespread damage and inflammation. Complications sometimes included phthisis (very low intraocular pressure, causing shrinking of the eye); inflammation inside the eye; sympathetic ophthalmia (inflammation in the other eye); hypotony; and occasionally, chronic pain following the procedure. These severe complications made TSCPC a treatment that most practitioners reserved for severe, end-stage glaucoma patients with poor visual potential.
MicroPulse was developed in the 90s to perform focal laser for macular edema, to treat leakage from retinal vessels while minimizing collateral damage. The technology was then adapted to be used for cyclophotocoagulation, to treat the ciliary body and lower IOP while similarly minimizing damage to adjacent structures. While the earlier cyclophotocoagulation technology applied the laser as a continuous wave, MicroPulse is essentially a subthreshold laser that chops the continuous-wave pulse into short pulses. This allows the tissue to heat and cool and heat and cool, preventing the thermal ramping-up that leads to increasing temperature and tissue destruction. Multiple studies, done both in vivo and on cadaver eyes, have demonstrated that this results in minimal changes to the adjacent tissue.
The current version of MicroPulse uses a 31.3-percent duty cycle, which means that 31.3 percent of the time the laser is being administered; the remainder of the pulse, the laser is off. This translates to 0.5 milliseconds of “on” time and 1.1 ms of “off” time, using one-third as much energy as when the laser is applied in a continuous wave.
This version of the laser causes far fewer adverse effects than the continuous wave treatments. There are minimal changes to the tissue surrounding the ciliary body, compared to TSCPC. A temporary mydriasis can happen, but sympathetic ophthalmia and phthisis are virtually never reported. Overall, it’s clear from the literature that severe complications are very uncommon with this version of the technology.
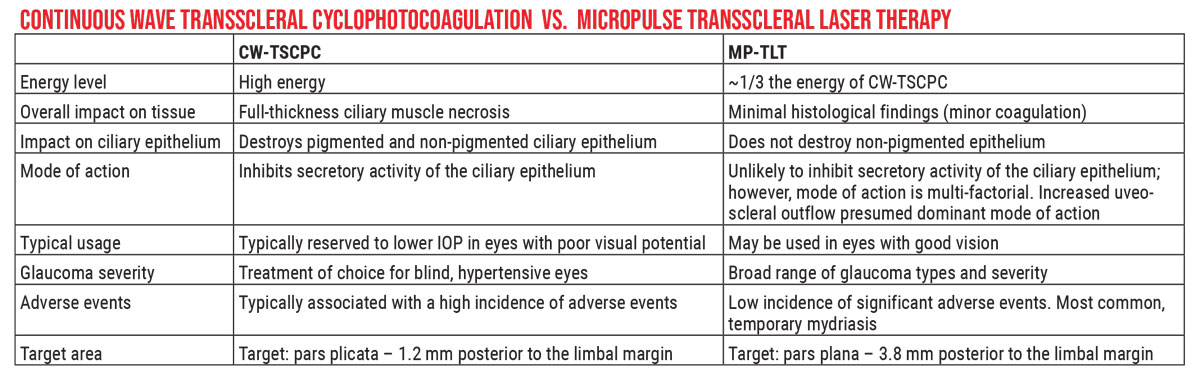 |
| Unlike the earlier continuous-wave laser, which was designed to partially disable the ciliary body via thermal energy, the MicroPulse Transscleral laser achieves a lower IOP while only causing minimal tissue damage. The mechanism of action remains unclear. Click image to enlarge. |
Unresolved Questions
There are still some issues being resolved. Because the tissue doesn’t heat up as much and cause obvious damage, it’s a little harder to explain why MicroPulse is effective at lowering IOP. The lower energy doesn’t completely inhibit the secretory activity of the ciliary epithelium and ciliary body, which suggests that there must be other ways in which it works to lower eye pressure.
There are multiple theories that attempt to explain what the laser energy is doing that ends up lowering the IOP. One theory is that it causes inhibition of secretory activity, much like TSCPC, but to a lesser degree. Other proposed theories include that MicroPulse increases uveoscleral outflow, and that it has a pilocarpine-like effect, which increases the trabecular outflow due to a posterior displacement of the scleral spur.
It’s not yet clear if one of these explanations is more correct than the others, but it’s possible that all of these mechanisms are working together. If this turns out to be true, then a MicroPulse treatment could be seen as similar to a combination glaucoma drop—increasing uveoscleral and trabecular outflow, while decreasing aqueous production.
Another issue that still needs to be resolved is refining the variables involved in applying the treatment. Three main variables can affect the impact of the procedure: the total power used, the total duration that laser is applied to the eye, and the velocity at which the laser is swept over the tissue (“dwell time”). The importance of the last factor can be compared to sweeping your hand over a lit candle: If you do it quickly, the impact on your hand will be minimal, but if you move your hand over the candle very slowly, you’ll get burned. I’m very interested in adjusting these variables to help understand what will make the surgery more efficacious.
Unfortunately, many studies involving the current MicroPulse procedure don’t report the amount of time spent in a given quadrant or hemisphere, and even fewer report the sweep velocity or dwell time. These factors haven’t been on people’s radar as important variables; surgeons tend to think that if the patient’s glaucoma is more severe they should simply increase the power. We’re not routinely considering variables such as pigmentation of the ciliary body—or as a correlate, trabecular pigment—or the exact positioning of the ciliary body. This is one reason the ideal parameters for use remain to be determined.
How I Use It
Although MicroPulse can be used outside of the OR with minimal anesthesia, I tend to use it in the OR because I find it makes the procedure technically easier, and it’s more comfortable for the patient. I want to be precise about the orientation and the timing of my sweep to ensure the effectiveness of the procedure. It’s undesirable to have the probe sliding anteriorly onto the cornea or posteriorly due to patient movement, because you can inadvertently treat the incorrect area. (Doing so probably wouldn’t hurt the eye, but it could make the treatment less effective.) Therefore, I prefer to do this in a controlled setting like the OR, and sedate the patient a fair amount.
I know that some surgeons are able to perform this procedure in the office using retrobulbar anesthesia, but in my early cases under Monitored Anesthesia Care (MAC) anesthesia with a retrobulbar block, I found my patients were stimulated by the laser, causing them to move intraoperatively. I currently use a strong “cocktail” of IV anesthetics, including propofol and ketamine, which adequately sedates the patient and has eliminated the need to do a retrobulbar. One advantage of this technique is that I don’t need to patch the patient post-procedure.
My typical protocol when using the new handpiece is a power setting of 2,500 mW, spending 50 seconds per hemisphere, and 20 seconds per sweep. (I routinely note these factors in my operating log for future reference.) I don’t like to use a lid speculum during the procedure, because I find that the MicroPulse handpiece can get hung up on the speculum and impede the sweeping motion. I find that the newer MicroPulse handpiece is effective at pushing the eyelid out of the way.
Patients for whom I find MicroPulse particularly useful include:
- patients who’ve had prior glaucoma surgery, such as an ExPress shunt, or a tube or trabeculectomy. When a patient already has a tube shunt and is still progressing, one option would be to implant a second tube, but most glaucoma specialists will tell you that a second tube is not their favorite option, because there’s already significant hardware in the eye. MicroPulse has been a great adjunct for patients in this situation.
- patients who are poor incisional surgery candidates. This would include those in a nursing home, those who aren't cooperative, and any patient whose circumstances cause you to be concerned about infection.
- patients in whom there’s good reason to want to do both eyes at the same time. MicroPulse can treat both eyes in a single surgery without any additional risk or lesser outcome. For example, if I’m doing an exam under anesthesia and find both eyes have high pressure, I can simply treat both eyes and be done. (I put this possibility in my consent form.)
Of course, MicroPulse does have limitations. For example, given that MicroPulse is a good adjunct to an existing tube shunt, one might wonder whether it could be used instead of a primary shunt. A recent paper asked this question, comparing MicroPulse to initial Ahmed valve placement.1 At 12 months, 73.3 percent of those in the Ahmed group had pressure lowered by 30 percent or more; this level of pressure-lowering was only achieved in 33.3 percent of patients receiving MicroPulse. The number of drops being used was reduced significantly in both groups, but was reduced more in the Ahmed valve group. Half of the eyes in the MicroPulse group were referred for additional treatment because of acute postoperative IOP rise, while no further procedures were necessary in the Ahmed shunt group. So according to this study, MicroPulse wouldn’t be best initial treatment option if a tube is a possibility.
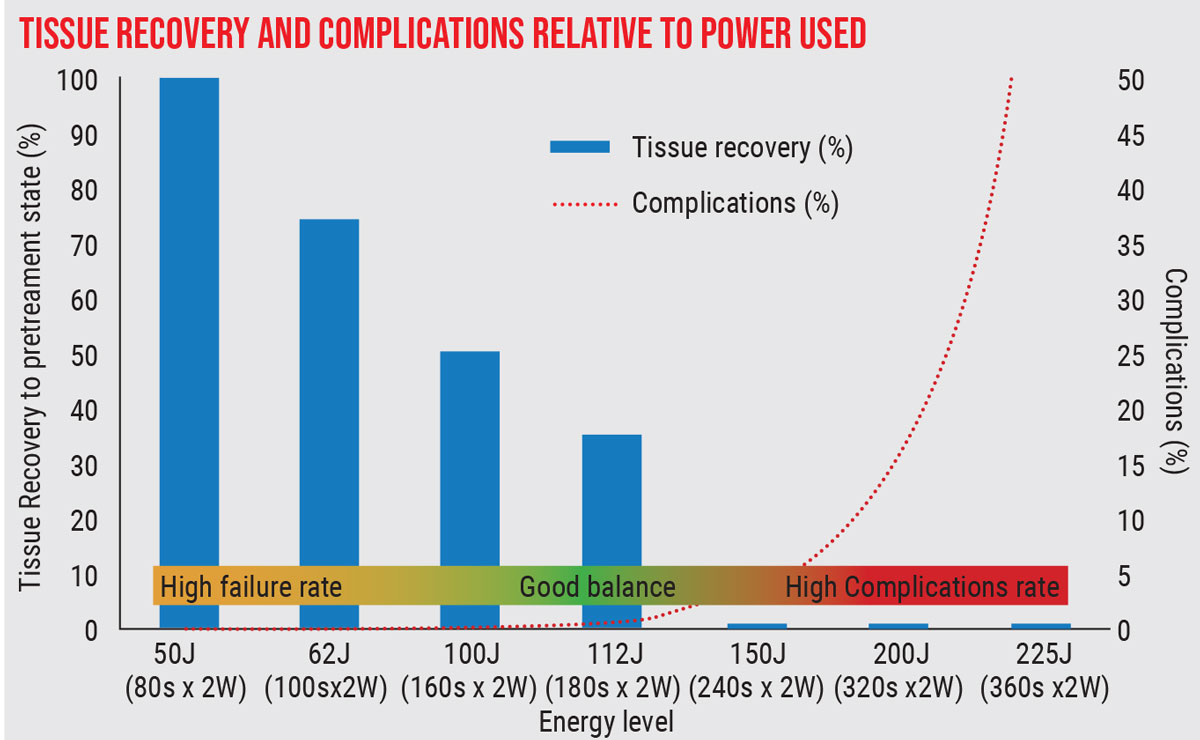 |
| Ideal parameters for MicroPulse are still being worked out. This study showed that at low energy levels, need for retreatment is high, while at high energy levels, complications increase dramatically. (Sanchez et al, 2018).8 Click image to enlarge. |
Starting Cautiously
As noted, most surgeons currently reserve MicroPulse for end-stage, refractory eyes. In fact, the first two studies of this technology were conducted on patients with refractory glaucoma by Paul Chew, MD, and Maria Aquino, MD. The first study treated 38 patients with refractory glaucoma. It found a success rate of 80 percent—defined as a final IOP between 6 and 21 mmHg or a 30 percent IOP reduction—at final follow-up.2 No patients experienced hypotony or vision loss. Seven patients (18 percent) reported mild pain on postop day one, but the remainder reported no postop pain.
The second study involved 48 patients with refractory end-stage glaucoma that were randomized either to continuous-wave treatment (TSCPC) or micropulse.3 The micropulse did better in terms of success rate (52 percent vs. 30 percent at 18 months). Mean IOP was reduced 45 percent in both groups, but the complication rate was higher with the continuous wave treatment.
Initially, like many surgeons, I reserved use of this procedure for patients who were not ideal surgery candidates and patients who needed to have both eyes treated at the same time. Gradually, however, I began trying it on a broader range of patients, with some success. I discussed my broadening criteria for treatment—treating patients with good central vision—with my colleague Syril K. Dorairaj, MD, from the Mayo clinic in Jacksonville, Florida. After comparing our criteria we decided it might be worth looking at both sets of data.
We pooled our data and looked at the MicroPulse patients who started with 20/40 vision or better.4 (Our laser settings and techniques were similar.) We looked at patients out to 12 months and found significant IOP-lowering and reduction in use of medications. On the downside, 12.5 percent of these individuals, at final follow-up, had lost two or more lines of vision. Those experiencing this outcome were not random, however. A majority of these patients were phakic, and the data suggested that the vision loss was attributable to cataract formation. My take-home was that this laser should be used with caution in phakic patients due to the risk of cataract formation. (It’s worth pointing out that if a patient has advanced glaucoma and the alternative is a trabeculectomy, the patient is also likely to get a cataract from having that procedure.)
In terms of potential complications, published studies reveal that chronic uveitis, cataract formation and macular edema are seen in some patients.5,6 On the other hand, I haven’t observed phthisis, corneal edema or persistent hypotony in my experience. (Some of the studies that do cite these complications used pretty high levels of laser energy.)5,7,8
Expanding the Scope
Today the indications for MicroPulse are broadening even further. Our group’s retrospective 2019 study demonstrated that MicroPulse was effective and relatively safe in patients with good central vision.4 In addition, many other papers have described using MicroPulse to treat different kinds of glaucoma, including open-angle glaucoma, exfoliative glaucoma and uveitic glaucoma. The published data indicate that it’s safe and effective for many different kinds and stages of glaucoma. In short, the evidence is mounting that this is not something that needs to be relegated only to people who have poor visual potential.
A few examples:
• A retrospective study published in 2020 looked at the outcome of using MicroPulse on 342 eyes of 214 patients with a wide spectrum of glaucoma-related issues, including ocular hypertension, all severity levels of glaucoma (including eyes with good vision and treatment-naïve eyes), and all types of glaucoma, include normal-tension glaucoma.9 At one year, 67.8 percent had achieved a 20-percent-or-greater IOP reduction. The data showed that the amount of IOP reduction was greater when the starting IOP was higher, and the chosen laser power setting also correlated with the amount of IOP lowering. (IOP dropped an average of 31.5 percent with the laser power set at 2,500 mW or more, and 17.8 percent when the power level was less than 2.500 mW, p<0.02.) No patients demonstrated persistent inflammation or hypotony, phthisis bulbi, or sympathetic ophthalmia. Interestingly, the mean number of topical glaucoma medications was unchanged from baseline to one year.
• Also in 2020, Rob Noecker, MD, and colleagues reported on a retrospective study looking at 95 patients with various types of glaucoma who were refractory to topical drops and poor candidates for incisional surgery.10 Mean preop IOP was 25.1 ± 5.3 mmHg; mean postop IOP at 12 months was 17.5 ± 5.1 mmHg (p=0.004). Mean number of medications dropped from 3 ±1.1 preop to 1.4 ±1 at one year (p=0.03). Seventy-three patients (77 percent) achieved success with one treatment; the remaining patients achieved significant IOP-lowering after one to four additional treatments. There were no instances of prolonged intraocular inflammation or long-term hypotony.
Special circumstances such as glaucoma in post-keratoplasty patients and pediatric glaucoma have also been studied with MicroPulse:
• A 2019 retrospective study of 61 post-keratoplasty eyes of 57 patients that had received MicroPulse treatments (31 eyes received a single treatment; 21 received two treatments; eight eyes receive three; and one eye received four treatments) found that it reduced mean IOP significantly out to one year.11 Six eyes (10 percent) received subsequent glaucoma filtration surgery. Notably, graft survival was 94 percent at one year and 81 percent at two years after the initial laser treatment.
• A prospective study published in 2019 included 45 eyes of 36 children requiring TS-CPC; it compared the outcomes of MicroPulse vs. continuous-wave applications.12 Success was defined as an IOP of 5 to 21 mmHg at six months, with no vision-threatening complications. IOP reduction was 63 percent in the MicroPulse group and 67 percent in the continuous-wave group. The success rate was higher in the MicroPulse group (71 percent vs. 46 percent), but the difference wasn't significant. However, while no significant complications were noted with MicroPulse, one eye in the continuous-wave group developed phthisis bulbi, and two eyes had severe pain and uveitis.
Into the Future
It’s true that much remains to be determined regarding how best to use this technology. We still need more prospective trials to determine what the most effective settings for patients are. Furthermore, we need standards for the sweep velocity, which may have a significant impact on how effective the treatment is. We could even consider applying the treatment in discrete spots, similar to TSCPC, to standardize the application further. And, we have yet to determine what types of glaucomas are more successfully treated with MicroPulse and what types of patients have better IOP-lowering (patients with prior glaucoma surgery, or those who haven’t had glaucoma surgery). These are questions we need to work on, to standardize the dosage and help everyone use this procedure more effectively.
Nevertheless, our experience, and that of many other surgeons, suggest that this technology could be benefitting far more patients than it currently is. I hope that other surgeons will help to expand these horizons.
References:
1. Fili S, Kontopoulou K, Vastardis I, et al. Transscleral cyclophotocoagulation with MicroPulse laser versus Ahmed valve implantation in patients with advanced primary open-angle glaucoma. Int Ophthalmol 2021;41:4:1271-1282.
2. Tan AM, Chockalingam M, Aquino MC, Lim ZI, See JL, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol 2010;38:3:266-72.
3. Aquino MCD; Barton K, Tan AM, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol 2015;43:1:40-6.
4. Varikuti VNV, Shah P, Rai O, et al. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma 2019;28:10:901-905.
5. Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: Initial results in refractory glaucoma. J Glaucoma 2017;26:8:726-729.
6. Williams AL, Moster MR, Rahmatnejad K, et al. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma 2018;27:5:445-449.
7. Kuchar S, Moster MR, Reamer CB, Waisbourd M. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Med Sci 2016;31:2:393-6.
8. Sanchez FG, Peirano-Bonomi JC, Grippo TM. Micropulse transscleral cyclophotocoagulation: A hypothesis for the ideal parameters. Med Hypothesis Discov Innov Ophthalmol 2018;7:3:94-100.
9. Kaba Q, Somani S, Tam E, Yuen D. The effectiveness and safety of micropulse cyclophotocoagulation in the treatment of ocular hypertension and glaucoma. Ophthalmol Glaucoma 2020;3:3:181-189.
10. Nguyen AT, Maslin J, Noecker RJ. Early results of micropulse transscleral cyclophotocoagulation for the treatment of glaucoma. Eur J Ophthalmol 2020;30:4:700-705.
11. Subramaniam K, Price MO, Feng MT, Price FW Jr. Micropulse transscleral cyclophotocoagulation in keratoplasty eyes. Cornea 2019;38:5:542-545.
12. Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. J Glaucoma 2018;27:10:900-905.
Dr. Sieminski is an associate professor and director of glaucoma at the Ira G. Ross Eye Institute, University at Buffalo/State University of New York. She receives research support from Iridex.
Counterpoint: Expanding MPTLT Use is Premature
Despite showing promise, there are a number of reasons we shouldn't be too quick to use this technology on healthier eyes.
By: Ian P. Conner, MD
I’m well-acquainted with the MicroPulse procedure; I use it for many of my patients. In fact, my personal opinion is that we probably don’t use it as often as we should. However, it’s still a relatively immature technology; there’s still a tremendous amount that we don’t know about it. For that reason, I see plenty of reason to exercise care when considering expanding its use.
Here are several reasons we should proceed with caution.
• We’re still not sure how it works. It’s true that MicroPulse has been shown to cause minimal changes to adjacent tissue—a significant advantage over previous modalities. However, as Dr. Sieminski has noted, what’s actually causing the lowering of intraocular pressure remains unclear. Rushing to use a procedure in patients who may not be in danger of going blind from their disease—such as patients with earlier-stage glaucoma—is a questionable premise when we don’t even really know how the procedure works.
It’s one thing to treat patients with a poorly understood technology if we know they’re going to go blind unless we do something. This rationale is often used in other medical areas such as oncology; when we’ve tried everything that we understand and nothing has worked, then we try experimental protocols. Some would argue that expanding the use of MicroPulse to include early glaucoma patients or glaucoma suspects is a little bit like uncontrolled experimentation.
• The ideal parameters for different eyes haven’t been determined. Because we don’t really understand how it works, we don’t know what the optimal parameters are, in terms of the amount of energy being applied, how long we should apply it, the length of each pulse and how fast we should sweep the probe across the eye. Working out these parameters with patients who have very mild disease raises some ethical concerns.
• Even “minor” side effects can be a big deal to a patient with good vision. My experience confirms Dr. Sieminski’s point that side effects of MicroPulse treatment are limited. More severe complications like phthisis, corneal edema and hypotony are very rare, and that can be seen as an argument for using MicroPulse in a broader swath of patients. However, potential complications like cataract progression, transient macular edema and uveitis are still concerning in a patient with mild disease. Yes, issues like secondary cataract formation have also been reported with alternative treatments such as trabeculectomy in the past, but any loss of vision in a patient with good vision is worrisome. Likewise, having temporary mydriasis and blurry vision—which we don’t have a good treatment for, and which can last for months after the procedure—is not a benign condition, so we should think twice about subjecting patients to that.
If we’re going to use this treatment in patients with much milder disease, we need to think hard about these potential complications. We may consider them to be mild, but the patient might not agree. (At the least, we need to make sure that the patient understands that this is a possibility in the consenting process.)
• Many of the studies supporting the expansion of MicroPulse to less-sick eyes are retrospective. It's true that increasing numbers of papers are being published about using MicroPulse to treat different kinds of glaucoma. However, almost all of this data is retrospective. A surgeon, for example, may decide to start using MicroPulse to treat patients with pseudoexfoliation or uveitic glaucoma, and then publish the results.
Unfortunately, there’s a huge selection bias at play in this kind of study. These are not rigorous, controlled trials. In fact, it’s very uncommon for anyone to publish negative data from a series of patients treated with the expansion of a clinical device or procedure; there’s a bias toward publishing cases with good outcomes. For that reason, we need to be cautious about basing our decisions on retrospective data.
It’s reassuring that people are doing this and getting good results, but right now it’s far from a scientific certainty that expanding the indications for this treatment is a safe thing to do.
• We don’t know much about repeating MicroPulse treatments. The idea that patients might need more than one treatment is something we have to mention in patient consent forms, because up to half of the patients treated with MicroPulse require a retreatment at one year. So this is far from being a “one-and-done” treatment.
This is reminiscent of selective laser trabeculoplasty. When we treat patients with SLT, a certain proportion of them will need retreatment within a relatively short amount of time. However, SLT has been around for so long that we have a really good handle on the nearly nonexistent side effect/complication profile. As a result, we feel comfortable treating people with early disease using SLT—and treating them more than once or twice if we have to. In contrast, with MicroPulse, we have some data about treating patients once, but have almost no published data about patients who are treated two or three times.
The reality is, we just don’t know what the potential ramifications of multiple treatments might be in patients with good vision and mild disease. So it’s not necessarily reassuring to say that we can just repeat the laser if the effect wears off after a short period of time.
Caution is Warranted
All of these points have to do with the uncertainty of a relatively new procedure. Most of us are pretty comfortable offering new technologies with uncertain side-effect profiles in patients who are in dire need of treatment, but we should be a little more cautious about patients who aren’t likely to lose their vision.
Certainly there are circumstances in which MicroPulse compares favorably to other options. For example, I agree that patients who are post-keratoplasty have limited alternate options for controlling their IOP. The graft causes the glaucoma, but then many options for treating the glaucoma—for example, placing a tube in the eye—lead to graft trauma and/or failure, which is pretty devastating. However, even in this situation the use of MicroPulse warrants some caution because we don’t have a lot of data; we don’t know what’s going to happen to these grafts over the long term. Use of MicroPulse in children also appears to be possible, with the data so far suggesting no significant complications in the short term. But these patients may live for another 50 or 70 years, and we have no way of knowing what we may be signing them up for in the future. That data doesn’t currently exist.
In reality, MicroPulse is probably not a procedure for everybody. Whenever a new technology appears, there’s always a lot of initial enthusiasm. Then, as time goes by and we use it in our patients and published data appears, we start to whittle down the indications. Today, for example, I can list 19 specific criteria that I really like to be in place before I do a trabeculectomy. If the patient checks all of those boxes, I feel confident he or she will do really well; if not, then I have a sinking feeling the surgery may fail, because we know that about 50 percent of trabeculectomies fail within 5 years, no matter who does the surgery. But we’re nowhere near that level of understanding when it comes to MicroPulse.
This is a story that gets repeated over and over. We want a magic bullet, and we have a lot of hope that whatever the newest thing is might be that bullet. Then, as we use it, we find that it works really well in some patients, but patients have to have specific criteria to fall into that group.
MicroPulse will clearly have a place in our glaucoma armamentarium, but it will take some time to clarify exactly what that place should be.
Dr. Conner is an assistant professor of ophthalmology and bioengineering at the University of Pittsburgh. He serves as the chief of ophthalmology at UPMC Shadyside and the UPMC glaucoma fellowship director. He reports no relevant financial ties to anything discussed in this article.




