 |
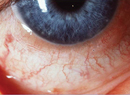
| The properties of hyaluronic acid hold huge potential for its use as a lubricant, cell matrix and tissue filler. |
Drug Delivery, A Central Theme
The eye is a unique venue for the pharmacologist: while it affords easy access with topical medications, it’s still surprisingly difficult to deliver sufficient concentrations of therapeutics, especially when the need is for extended durations. Exploration into new methods of drug delivery for the treatment of ocular disorders has focused on this limitation, and has taken advantage of an explosion in research into the formulation and manufacture of biomaterials and other delivery-device technologies. A prime example of this is the chemical modification of biopolymers, including collagens and hyaluronic acids for use in drug delivery and for stem cell propagation.1 Depot forms of a number of drugs have been brought to market in recent years, and it looks like this trend will continue. This year at ARVO, the search for new methods of drug delivery designed to increase efficacy, reduce side effects and increase patient convenience was highlighted.
Several presentations came from Jade Therapeutics, including reports on potential applications of their thiolated carboxymethylated hyaluronic acid films. CMHA chemistry has the advantage of flexibility, both in terms of its physical properties and its delivery potential, since the gels can be easily inserted on-site by a physician. Drug release from the CMHA matrix largely depends on the degradation of the matrix by hyaluronidases (HAase) present in ocular tissue. (Bowen RC, et al. ARVO E-Abstract 1295) reported on levels of HAase in ocular tissues; information that is pivotal to future optimization of CMHA matrices for ocular drug delivery. Another report (Lee WY, et al. ARVO E-Abstract 4139) investigated different formulations of the CMHA matrix and found that utilizing cross-linker Poly (ethyleneglycol) diacrylate created a film with favorable tensile strength, relaxation modules, durability and flexibility.
Another presentation examined use of CMHA matrices for delivering therapeutic proteins. (Wirostko B, et al. ARVO E-Abstract 262) In a model of corneal wound healing, the CMHA film was found capable of delivering recombinant human growth hormone in a controlled, sustained manner both in vitro and in vivo over a course of days to months. The CMHA matrix shows promise for alternative drug-delivery systems that reduce frequency and increase efficacy of a number of therapeutics for ocular disease. Another similar delivery system involved the use of collagen hydrogel implants, in which vancomycin was successfully delivered for prevention of postoperative ocular infection. (Mondal D, et al. ARVO E-Abstract 4135)
Hydrogel technology was employed in implanted devices or microparticles. One study used microparticles to deliver a glaucoma therapeutic (OHR1031; Ohr Pharmaceutical, San Diego), testing the release kinetics of the drug in vitro. (Malavia N, et al. ARVO E-Abstract 1296) Notably, these investigators utilized a dissolvable hydrogel that should yield sustained delivery without the need to recover an empty device. Another implant currently in preclinical evaluations is the ENV905 (Envisia Therapeutics; Research Triangle Park, N.C.), used for delivery of difluprednate for treatment of inflammation and pain associated with ocular surgery. (Garcia A, et al. ARVO E-Abstract 5897) The ENV905, which can be placed subconjunctivally or intracamerally, demonstrated in a rabbit model a robust reduction in corneal inflammation over four weeks. This type of delivery would eliminate the need for topical multidose therapies of extended duration.
An interesting application of hydrogel technology utilizes “nanowafer” drug delivery systems. (Acharya G, et al. ARVO E-Abstract 5032) In a model of corneal neovascularization, the nanowafer, a transparent disc containing nanoreservoir arrays of the small molecule tyrosine kinase inhibitor Axitinib, was demonstrated to be significantly more effective than b.i.d. drops.
Soft contact lenses are also being used for drug delivery. Using a novel 3-D in vitro eye model that mimics physiologic tear flow across the ocular surface, one study confirmed that commercial contact lenses can maintain a sustained drug-release profile for up to 24 hours. (Phan C-M, et al. ARVO E-Abstract 3085) Researchers also reported the successful application of dexamethasone-eluting contact lenses for the treatment of ocular inflammation in a rabbit model. (Ciolino J, et al. ARVO E-Abstract 148)
Two studies at ARVO 2015 presented new technologies to address the issue of patient compliance in clinical trials. A smartphone-based system for capturing images of conjunctival redness showed a high degree of correlation with measurements made by clinicians. (Corcoran P, et al. ARVO E-Abstract 3045) Another study evaluated how the shape of an eye-drop bottle dictates patient compliance by utilizing a novel eye drop application monitor. The monitor creates a log of the number of drops dispensed and how many landed in or outside of the eye. (Allen M, et al. ARVO E-Abstract 1919)
AMD and Anti-VEGF Delivery
An astonishing amount of research is dedicated to improving the treatment options and visual outcomes of age-related macular degeneration. While vascular endothelial growth factor inhibitors have begun to provide a means of holding off the permanent damage to vision that AMD causes, the necessity of once- or twice-monthly intravitreal injections is a financial and medical burden. Now, however, novel delivery systems are evolving to address this limitation. One fascinating approach involved a system of de novo designed cell-penetrating peptide constructs fused to a therapeutic protein transduction domain. (DeCogan F, et al. ARVO E-Abstract 4147) This system was shown to successfully transport microgram quantities of macromolecules, such as the very large monoclonal VEGF inhibitors, in a topical eye drop that enables penetration all the way to the posterior segment. Other studies focused on slow-release depots of VEGF-inhibitors. Initial in vitro pharmacokinetic work is being done on controlled-release polymer reservoirs of ranibizumab. (Abe T, et al. ARVO E-Abstract 4146) A non-invasive electroosmotic delivery of bevacizumab also proved successful and analogous to the efficacy of intravitreal bevacizumab. (Molokhia S, et al. ARVO E-Abstract 2293) A polymer nanoparticle system was described in which conjugation of bevacizumab was shown to reduce leakage of the drug into the bloodstream. (Kong L, et al. ARVO E-Abstract 5029) This prolonged its retention in the vitreous, making it potentially safer and more effective by reducing systemic exposure and extending ocular residence time. A number of in vitro and in vivo pharmacokinetic studies examining properties of becacizumab-packaged microparticles were presented, including PK studies of solid-state microparticles within sustained-release hydrogel matrices in both primates and non-primate species. (Tully S, et al. ARVO E-Abstract 222; Owens G, et al. ARVO E-Abstract 236; and Verhoeven RS, et al. ARVO E-Abstract 230) If this year’s presentations are any indication, a major focus of retinal therapies in the near future will be the refinement of these therapeutic delivery options.
Steroid therapy for retinopathy has not fallen by the wayside, however. A dexamethasone intravitreal implant together with macular grid laser was found effective for retinal vein occlusion (Massaro D, et al. ARVO E-Abstract 3752), allowing clinicians to extend the time between injections. PRINT technology was used to create biodegradable implants and microparticle suspensions of steroids for six-month, slow-release depots for intravitreal delivery. (Das S, et al. ARVO E-Abstract 4165) Release kinetics of loteprednol in a nanoparticle gel was also presented. (Hirani A, et al. ARVO E-Abstract 5038) Lastly, topical dexamethasone γ-cyclodextrin nanoparticle eye drops improved visual acuity and decreased macular thickness in patients with DME. (Ohira A, et al. ARVO E-Abstract 2289)
With regard to new therapies, effort has been focused on small-molecule therapies to avoid the problems of penetration and absorption of the macromolecule monoclonal VEGF inhibitors. Initial in vitro screens included multiple small-molecule, vitamin D receptor agonists (Merrigan S, et al. ARVO E-Abstract 158); combinations of inhibitors of the P13K/Akt/mTOR pathway (Sasore T, et al. ARVO E-Abstract 2305); and an assessment of an isoquinolone sulfonamide derivative that inhibits protein kinase function. (Sugimoto M, et al. ARVO E-Abstract 150) In models of neovascularization, intravitreal and systemic IL-18 immunotherapy was effective. (Campbell M, et al. ARVO E-Abstract 4803) Pharmacokinetics and initial vascular leakage models were used to successfully assess a topical receptor tyrosine kinase inhibitor formulated via mucus-penetrating particles that allow for enhanced penetration and activity in the back of the eye. (Schopf L, et al. ARVO E-Abstract 2279)
Two studies on regorafenib eye drops, which would be a breakthrough topical therapy, presented positive results using this multi-kinase inhibitor. (Beottger MK, et al. ARVO E-Abstract 2294; Klar J, et al. ARVO E-Abstract 246) SH-11037, a homoisoflavanone synthetic derivative of cremastranone, significantly suppressed angiogenesis in a murine model. (Sulaiman RS, et al. ARVO E-Abstract 2470) Tetramethylpyrazine, a unique small-molecule inhibitor of the chemokine receptor CXCR4 was also found effective. (Zhuang J, et al. ARVO E-Abstract 2465) Similarly, oral dosing of a chemokine receptor 3 (CCR3) antagonist effectively suppressed spontaneous neovascularization in mice and laser-induced injury in primates. (Ng Q, et al. ARVO E-Abstract 2290) Oral docosahexaenoic acid supplements protected against neovascularization and retinopathy in rat models of AMD (Ogami S, et al. ARVO E-Abstract 2350), and a new RNAi-based agent appears to inhibit both the angiogenesis and fibrosis promoted by periostin. (Nakama T, et al. ARVO E-Abstract 2280) A similar strategy was assessed using the anti-pigment derived growth factor aptamer Fovista and aflibercept together in a mouse model. (Walsh B, et al. ARVO E-Abstract 2298) A telomerase-derived peptide, GV1001, inhibited neovascularization in a rat model in a dose-dependent fashion. (Lee EK, et al. ARVO E-Abstract 2291) The variety of new agents showing promise in preclinical studies bodes well for treatment of retinal proliferative disease.
Clinical Trial Results
Dry AMD continues to be one of the main ocular disorders without significant Food and Drug Administation-approved therapies.2 In the clinic, BAM114341, a therapy designed to block formation of β-amyloid deposits seen in Alzheimer’s disease is being tested in a Phase II study in geographic atrophy secondary to AMD (Shearn SP, et al. ARVO E-Abstract 2840). The authors published the results of the four-month run-in period, in which fundus photography and autofluorescence were used to define pre-specified and patient-specific growth rates of lesions as the unique primary endpoints in this clinical trial. It will be exciting to see the efficacy results of this study, given recent studies linking β-amyloid formation in AMD with other neurodegenerative disorders of peptide misfolding such as Alzheimer’s disease.
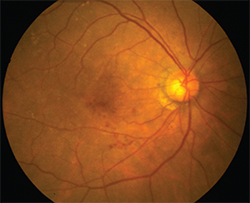 |
| Experimental AMD treatments at ARVO include radiation therapy and viral vectors. |
Results from the Phase I GEM study were reported to be successful. (Chandler S, et al. ARVO E-Abstract 2284) This first-in-human application of a subretinally injected lentiviral vector (RetinoStat) met the primary endpoint, was safe and well-tolerated and patients showed signs of clinical benefit. A humanized single-chain antibody fragment, RTH258, has been tested in the clinic in patients with AMD. This molecule has the advantage of allowing microvolume injections or infusions of 10 µl, leaving room for additional simultaneous treatments or permitting a sustained delivery platform.
Two mineralocorticoid receptor antagonists, spironolactone and eplerenon, were found effective for oral treatment of central serous chorioretinopathy, with the former showing superior efficacy for fast resolution of subretinal fluid and anatomic outcomes. Retinal branch occlusion and DME both received press coverage of their clinical trial results. The 52-week results of aflibercept in the VIBRANT study were positive for treatment of retinal branch occlusion. (Boyer DS, et al. ARVO E-Abstract 3749) Studies of DME therapy with aflibercept and bevacizumab showed positive results in pathologic myopia and persistent DME. (Nassaralla JJ, et al. ARVO E-Abstract 4622; Gillies MC, et al. ARVO E-Abstract 3144)
Cornea Corner
At ARVO, we always expect to see plenty of new studies in the area of corneal trauma, inflammation and wound healing. This year did not disappoint. Progress in gene-based therapy for inherited disease was demonstrated in a study of corneal clouding associated with MPS1, a condition known historically as Hurler’s syndrome. (Hirsch M, et al. ARVO E-Abstract 260) The syndrome is due to a genetic defect in iduronidase α-L, a key enzyme in glycosoaminoglycan metabolism. The study tested a viral-based expression of a replacement for IDUA in patient fibroblasts, mouse cornea and human cornea. The goal was to optimize the adeno-associated viral vector for corneal expression of IDUA, and that was demonstrated in all three test tissues. Stromal injections of optimized constructs into wild-type human corneas ex vivo yielded robust expression of the viral IDUA, providing hope for therapeutic intervention for corneal clouding in children with MPS1 and other varients of mucopolysacharride-associated disorders.
Chronic keratitis, whether from infection, trauma or surgical complication, remains a significant therapeutic challenge. As in years past, there was a wealth of studies, from neurotrophic keratitis to dry eye, aimed at improving therapeutic outcomes for those with corneal disease. One study tested the efficacy of the Rho-kinase inhibitor AMA0076 (Amakem NV, Belgium) in a rabbit model of corneal debridement. (Defert O, et al. ARVO E-Abstract 5610) Rho kinases are signal mediators for a host of cellular responses associated with cytoskeletal dynamics and motility.3 In the past, they have been explored as potential therapies for POAG. More recently their role in cellular inflammation and differentiation has been recognized, and this study aimed to test the ability of Rho kinases to enhance and accelerate the process of corneal wound healing. The study followed four days of healing for untreated animals, AMA0076-treated animals, and a positive comparator of recombinant growth hormone. The ROCK inhibitor and GH treatments were comparable for both re-epithelialization and resolution of corneal haze, and both were superior to control animals. Of note, AMA0076 did not cause the hyperemia that’s been associated with other ROCK inhibitors.
Currently, steroids are the primary treatment modality for ocular inflammation, but there is a significant need to improve upon existing treatment regimes and dosing strategies. Implants such as those tested in the Envisia Therapeutics study performed by Garcia, et al, discussed previously, are designed to provide a single-dose treatment via a subconjunctival or intracameral device. (Garcia, et al. ARVO E-Abstract 5897) This study tested ENV905, a biopolymer implant designed to deliver therapeutic dosing of difluprednate over a four-week taper, compared with q.i.d. Durezol in a rabbit model of postoperative inflammation. Using slit-lamp exams and Hackett-McDonald scoring, the implants were superior to both placebo implants and to topical steroid.
In a similar strategy, other researchers tested a weekly dosing regimen of cyclosporin delivered using mucoadhesive nanoparticles in a mouse model of dry eye. (Lui S, et al. ARVO E-Abstract 5036) This regimen enhanced anti-inflammatory efficacy relative to placebo or to higher doses of CsA.
It’s long been thought that dry eye was a condition that was most severe in winter months, but no studies had ever been done to test this. A group from our research firm Ora pooled data from 10 trials and identified 270 patients who had participated in at least one summer study (April to September) and one winter study (November to April), and were randomized to placebo groups in both. (Ousler G, et al. ARVO E-Abstract 4462) Both ocular discomfort and dryness symptom scores were significantly higher during the winter months, consistent with the prevailing wisdom. It’ll be interesting to see if clinical signs of dry eye such as corneal stain show similar patterns.
The search for new dry-eye therapies continues, and ARVO presented a mix of repurposed products, pipeline products and new chemical entities. One presentation described repurposing of the immunosuppressant rapamycin4 (Rapamune, Pfizer) as a therapy for Sjögren’s syndrome, using a non-obese mouse model as a test platform. (Shah M, et al. ARVO E-Abstract 4810) After a 12-week treatment of b.i.d. rapamycin, SS markers were all significantly reduced compared to placebo, including lacrimal lymphocytic infiltration and tear cathepsin S activity. Another preclinical trial tested the integrin antagonist GW559090 in a mouse model of dry eye. (Krauss AH, et al. ARVO E-Abstract 2472) Integrins are key players in lymphocyte activation and chemotaxis; lifitegrast, a drug for treatment of dry eye currently under FDA review, inhibits another member of the integrin family, lymphocyte function antigen 1.5
Another therapeutic approach gaining favor for corneal disease is stem-cell transplantation. One notable study used a mouse model of bacterial inflammation to demonstrate that the local delivery of mesenchymal stem cells is an effective and safe approach for immunomodulation and treatment of ocular inflammation. (Zhang R, et al. ARVO E-Abstract 942)
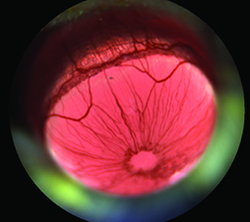 |
| Increases in vasodilation in mouse models of ocular inflammation can be quantified and used as efficacy measures in preclinical drug assessments. (Whitlock A, et al. ARVO E-Abstract 4886) |
Gene Therapies
ARVO always highlights the cutting edge of scientific research, so it was no surprise to see a number of presentations describing the latest progress towards genetically based therapies. One of these reports combined gene-editing CRISPR technology and genetically modified, induced pluripotent stem cells to generate 3-D retina reporter cell lines for the study of photoreceptors. (Wahlin KJ, et al. ARVO E-Abstract 3596) Several studies focused on utilizing gene therapy to target the oxidative stress that accompanies a number of retinal diseases. By utilizing a mouse model of retinal pigment epithelium oxidative stress, researchers demonstrated that delivery of genes for antioxidant enzymes can be used as a tool to reverse oxidative stress. (Biswal MR, et al. ARVO E-Abstract 3189) In contrast, a different study proposed that targeting of transcription factors regulating hundreds of genes that combat oxidative stress would be more effective than the delivery of antioxidant enzymes. (Xiong W, et al. ARVO E-Abstract 3188)
Technological improvements are always on display at ARVO. One example that appeared in several presentations was the automation of ocular redness measurements. (Rodriguez J, et al. ARVO E-Abstract 340; Finis D, et al. ARVO E-Abstract 4444) Ocular redness is a key diagnostic indicator in studies of dry eye, and these methods, particularly in combination with devices designed for patient documentation of redness described earlier, can be real game-changers in drug development for ocular inflammation. Two reports from Ora examined blinking under natural conditions using continuous monitoring technology. (Lane K, et al. ARVO E-Abstract 4447; Harmeling L, et al. ARVO E-Abstract 4486) These studies continue to advance our understanding of compensatory mechanisms in ocular surface disease.
Another presentation from researchers at Ora examined the differences between corneal fluorescein staining measurements made in research or clinical studies with those in a typical ophthalmic practice. (Angjeli E, et al. ARVO E-Abstract 336) While clinicians often visualize staining with a standard slit lamp, protocols used in clinical trials often employ filters, reduce extraneous wavelengths, minimize background light and significantly enhance the image’s signal-to-noise ratio. The study compared patient staining with and without low-pass filtration and showed that most measurements made with slit lamps alone underestimate the extent of staining.
As usual, there were far too many interesting discussions, presentations and studies at this year’s ARVO meeting to provide a comprehensive summary. Readers would do well to keep this in mind when planning for educational outings in 2016, since a succinct summary of the 2015 ARVO meeting, like those of meetings past, is simply, “You had to be there.” REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School.
1. Burdick JA, Prestwich GD. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv Mater 2011;23:H41–H56.
2. Buschini E, Fea AM, Lavia CA et al. Recent developments in the management of dry age-related macular degeneration. Clinical Ophthalmology 2015;9:563–574.
3. Yu OM, Heller Brown J. GPCR and RhoA-Stimulated Transcriptional Responses Mediating Inflammation, Differentiation and Cell Proliferation. Mol Pharmacol 2015 Apr 22. pii: mol.115.097857. [Epub ahead of print]
4. Cobbold S. The mTor Pathway and integrating immune regulation. Immunology 2013;140:391-398.
5. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5% for treatment of dry eye disease: Results of the OPUS-1 phase 3 study. Ophthalmology 2014;121:2:475.