There are few drugs, or classes of drugs, that can compare with corticosteroids in terms of their importance and utility. As a group they represent first-line therapy in the treatment of immune and inflammatory disorders, and are key components in many therapies for cancer. Corticosteroids are also essential treatments for conditions involving disruptions or decreases in a patient’s natural production of dihydrocortisol or aldosterone, the endogenous forms of these compounds.
In keeping with their vital role in physiological homeostatic regulation, it’s not surprising that the benefits of pharmacologic application of glucocorticoids come with several significant side effects. For example, steroid use, especially prolonged use, is associated with immunosuppression, bacterial super-infection, delayed wound healing and osteoporosis.1,2 These side effects are dependent on both dosage and duration of therapy, and there’s little doubt that they’re manifestations of the physiological actions of the hormone. Beyond these adverse actions, ophthalmologists have specific concerns when considering use of steroid therapy: While these powerful drugs have great utility for a number of ocular conditions, they’re also linked to effects such as ptosis, stromal haze, increases in intraocular pressure and development of posterior subcapsular cataract.3,4 The solution to this problem is to develop a selective or partial glucocorticoid receptor agonist that preserves the anti-inflammatory action of the native steroid while minimizing the undesirable effects. This approach has worked with drugs acting at the related estrogen receptor such as tamoxifen or raloxifene.5
In this column we provide a primer on steroid signal transduction, and then focus on ocular-specific pathways, including those involving adverse steroid effects. We’ll look at progress in developing new glucocorticoids and consider other approaches to therapeutic modulation of this critical signaling pathway.
Signal Transduction 2011
Ophthalmologists have a handful of choices when it comes to steroid therapy.6 For the most part, formulation (including combination formulations) dictates the specific choice of steroid used for indications such as uveitis, macular edema or allergy. Differences in pharmacokinetics also play a role; triamcinolone, for example, has become a popular choice for intravitreal injection because its hydrophobic character provides a long duration, “depot-like” effect.7 While studies have demonstrated differences in signal pathways regulated by specific drugs, the adverse steroid effects are exhibited by all of the available agents. In order to develop more selective compounds it’s necessary to tease out the complexities of steroid signaling.
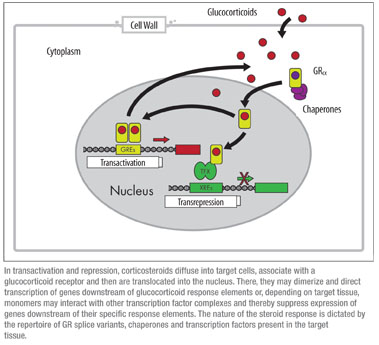
Most biology texts describe steroid signaling as a linear, stepwise process in which cortisol enters the cell through a passive diffusion and binds to its receptor. The receptor-ligand complex is then translocated from cytoplasm to nucleus where it interacts with glucocorticoid response elements to activate (or suppress) transcription of target genes. Of course, it’s not that simple—how could it be? In a recent report, microarray analysis of glucocorticoid-induced changes in lens epithelial cell gene expression showed more than 130 genes were up- or down-regulated at least twofold within 48 hours of dexamethasone treatment.8 Another study employing keratinocyte cell cultures from glucocorticoid receptor knockout mice compared with control cultures estimated that GR signaling regulated expression of approximately 442 different genes.9 Regardless of the specific number of genes or the particular target tissue, it’s clear that mechanisms underlying steroid signaling involve an exceedingly complex, multivariate network of positive and negative inputs.
To understand the complexity of this network, it’s necessary to first recall that the glucocorticoid receptor has a modular structure, comprising three functional elements—a modulatory (sometimes called a trans-activation) domain, a DNA-binding domain and a steroid ligand binding domain. Each of these domains provides a potential opportunity for pharmacological intervention. Intra-molecular contacts allow each domain to regulate the function of the others, while at the same time they can act with some degree of autonomy. The GR is derived from a single gene but several recent studies have established that there are two families of receptor splice variants, GRα (the “original” GR) and GRβ.10 Each of these families includes six or more alternatively spliced products, and all of the GRβ forms lack a functional ligand binding domain. Despite this, they are still capable of dimer formation and subsequent downstream modulation.11 Tissue-specific expression of these splice variants has yet to be fully explored, but it’s clear that this diversity in GR structures has the potential to direct a significant portion of the signaling specificity.
It seems that the two major signaling modalities, transactivation and transrepression, involve qualitatively different mechanistic steps.12 When genes are turned on during transactivation, steroid binds to the GR and the hormone-receptor complex enters the nucleus as a dimer, then recruits cofactors such histone acetylases and chromatin remodeling complexes to allow transcriptional machinery to access genomic DNA. This process involves the direct interaction between the ligand-bound GR and the glucocorticoid response element, or nucleic acid target sequence. Transactivation can be impacted by one or more cytosolic or nuclear cofactors which form receptor complexes with or without bound ligand.12 Tissue-specific expression of cofactors can alter the magnitude of transcriptional response, the sensitivity to steroid or both.13
The second type of steroid-regulation is transrepression, the selective reduction in target gene expression. This activity is often mediated by monomer GR-ligand complexes interacting with transcriptional regulatory factors such as NF-κB, AP1 or STAT5.14 Transcriptional inhibition requires protein-protein interactions involving the GR transactivation domain, but there is typically no direct interaction between the GR and any nucleic acid response elements. A key to understanding current approaches in the development of newer, more selective GR agonists is the observation that anti-inflammatory effects of glucocorticoids are mediated primarily by transrepression.15
 |
Transcriptional regulation of specific genes is the hallmark of steroid action. When a clear link between steroids and IOP was established, researchers began searching for steroid-regulated genes. The target was a structural protein that was originally dubbed the trabecular-inducible glucocorticoid response gene, or TIGR.16 This signaling pathway has since become one of the best-studied examples of any gene regulation-target triad. The gene product, a protein now referred to as myocilin, is induced in some inherited forms of juvenile primary open-angle glaucoma and also appears to be upregulated in some adult forms of POAG.17 While there are many different documented mutations in the myocilin gene associated with POAG, the phenotype is a consistent increase in overall myocilin expression. The question that remains, however, is one of cause and effect: Because the function of myocilin is unknown, it’s impossible to say what role it may have in the etiology of glaucoma. What is clear is that for some patients, steroids induce an excessive expression of myocilin, and, similar to those with inherited mutations in the myocilin gene, a buildup of myocilin protein in the trabecular meshwork is associated with elevated IOP and glaucomatous damage.
Several studies have used glucocorticoid-induction of myocilin as a model system of functional changes associated with POAG. One study looked at changes in the trabecular meshwork in a bovine model of prednisolone-induced IOP increases.18 The researchers documented accumulation of extracellular matrix plaques at the TM outflow loops and an increase in the TM basement membrane. In an earlier study, investigators examined expression of normal and mutant forms of myocilin;19 they showed that the wild-type protein is expressed in many different tissues; in the TM it’s resident in the intracellular endoplasmic reticulum and is also secreted into the aqueous humor. In cultured TM cells or cell lines, most mutant forms of myocilin accumulated in the ER and were not secreted; when mutant and native forms were co-expressed, the mutants exerted a dominant-negative effect preventing secretion of the wild type.20 Still other mutations displayed a preferential expression and secretion over the wild-type gene product. And despite this scrutiny, the myocilin gene locus represents only one of 13 genes mapped in pedigrees of autosomal dominant juvenile POAG.17 Perhaps the greatest value of myocilin currently is as a biomarker for steroid-induced increases in IOP and, down the road, as a tool for a mechanistic understanding of glaucoma etiology.
Another clue to the link between steroids and glaucoma comes from studies of the dynamics of actin expression. Several studies showed increases in TM stress fiber cross-links in glaucomatous eyes21 and in steroid-treated human TM cultures,22 suggesting that this commonality may somehow underlie the decreased aqueous humor outflow that occurs in POAG and in steroid-induced increased IOP. More recently, a study showed a similar increase in actin cross-link formation in stress fibers of the lamina cribrosa, both in dexamethasone-treated cultures and in glaucomatous eyes in situ.23 It’s not difficult to imagine such cross-links causing an increase in aqueous humor hydrostatic pressure by reducing outflow; in combination with this, cross-links at the lamina cribrosa may be causative for the damage to the optic nerve in POAG.
A second adverse effect of steroids in the eye is the development of posterior capsular cataract, a type of lens pathology that is nearly diagnostic of long-term, systemic glucocorticoid use.24 More recently, intravitreal steroid treatments have been approved for macular edema, branch retinal vein occlusion and other neovascular conditions. Another study showed that virtually all patients receiving intravitreal triamcinolone develop some clouding of the lens, and that this effect is both time- and dose-dependent.25
What’s the Solution?
New drugs targeting the steroid hormone signaling pathways must somehow minimize adverse effects despite administration in pharmacological, rather than physiological doses. There are a number of different approaches to this problem, including selective or partial agonists, allosteric modulators or “physiological congeners.”
For example, the estrogen receptor partial agonist raloxifene was developed to avoid the side effects associated with endogenous estrogen in hormone replacement therapy.5 Raloxifene has a different spectrum of binding affinities for estrogen receptors in different tissues and thus provides therapeutic benefits of estrogen while minimizing adverse effects of the endogenous hormone.
Another approach is exemplified by allosteric (from the Greek, meaning “other shape”) modulators, drugs that act on targets at sites other than the native ligand binding site. Such modulators commonly target neurotransmitter receptors or ion channels; lidocaine, for example, is an allosteric modulator of sensory neuron sodium channels and nicotinic cholinergic receptor channels.26 While no examples of glucocorticoid allosteric modulators are currently available, the numbers of endogenous co-factors and chaperones implicated in glucocorticoid signaling13 would suggest that this might be a likely avenue for future drug development.
The group known as physiological congeners includes any drug that can elicit similar effects as another. For glucocorticoid-invoked anti-inflammatory effects, an example of a congener would be any of the non-steroidal anti-inflammatories such as nepafenac, ketorolac or ibuprofen. Like corticosteroids, these drugs are effective against inflammation, but they act by a different mechanism, inhibition of cyclo-oxygenase, rather than regulation of gene transcription.1 Of course, these drugs are not without their own set of adverse-effect baggage—and they are certainly not as potent as steroids. Of these various approaches, the greatest efforts have focused on partial glucocorticoid agonists, a group of drugs referred to collectively as SEGRAS: selective glucocorticoid receptor agonists.27
SEGRAS Explained
A critical observation in the development of selective GR agonists involved a GR mutation in which receptor transactivation effects were lost while anti-inflammatory effects were retained.28 This result was the first evidence for multiple, distinguishable aspects of GR signaling, and provided the rationale for the development of partial agonists. Since then, there has been a concerted effort to identify compounds with a partial GR receptor agonism suitable for clinical development. A common paradigm for development is to use a two-step screen for transactivation (e.g., induction of gluconeogenesis), and transrepression (reduction in IL-6 protein levels); compounds that can preferentially elicit the transrepression are candidates for further study.29 A number of compounds have been identified using this approach and several candidates are in development. Clinical trials, including both Phase II (ZK 245186, for atopic dermatitis) and Phase III (BOL-303242-X, Mapracorat, for inflammation following cat-aract surgery) trials have been conducted or are under way.30
What we already know about the adverse ocular effects of steroids can provide essential clues to testing new compounds. For Mapracorat, pre-clinical studies went beyond IL-6 levels to demonstrate that it could also attenuate other inflammation-associated cellular signaling events, such as increases in IL-1β, IL-8 and TNF-α in human ocular cells, similar to either dexamethasone or triamcinolone.31 In corneal epithelial cells, this same partial GR agonist inhibits hyperosmolar-induced cytokine release and MAPK pathways, suggesting a potential use in the therapy of dry eye. And in cultured human TM cells, it’s been shown that Mapracorat increases myocilin expression and secretion levels by only half of that seen with either dexamethasone or prednisolone.32 The close association between steroid-induced elevation of myocilin and steroid effects on IOP suggests that compounds like Mapracorat may represent an important step in the development of newer, better ocular steroid therapies.
Dr. Abelson, a clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Dr. McLaughlin is a medical writer at Ora Inc., in Andover.
1. Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11th ed. New York: McGraw Hill, 2008.
2.Poetker DM, Rah DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin N Am 2010;43:753–768.
3. Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res 2009;88:752-9.
4. James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther 2007;23:403-20.
5. Firestone GL. Unraveling the intricacies of receptor activated cell signaling and transcriptional cascades enhance opportunities to develop new therapeutic targets for endocrine and metabolic diseases. Curr Opin Pharm 2010;10:607–612.
6. Edelman J. Differentiating intraocular glucocorticoids. Ophthalmologica 2010;224:1(S):25-30.
7. Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: The past, the present, and the future. Surv Ophthalmol 2008;53:139-149.
8. James ER, Fresco VM, Robertson LL. Glucocorticoid-induced changes in the global gene expression of lens epithelial cells. J Ocul Pharmacol Ther 2005;21:11-17.
9. Sevilla LM, Bayo P, Latorre V, Sanchis A, Perez P. Glucocorticoid receptor regulates overlapping and differential gene subsets in developing and adult skin. Molecular Endocrinology 2010;24:2166-2178.
10. Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol 2005;16:301-7.
11. Charmandari E, Choruses GP, Chino T, et al. The human glucocorticoid receptor (hGR) beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes. Mol Endocrinol 2005;19:1:52.
12. Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH. Glucocorticoids: Extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol 2010;224:311-315.
13. Luo M, Simons SS. Modulation of glucocorticoid receptor induction properties by cofactors in peripheral blood mononuclear cells. Human Immunol 2009;70:785-789.
14. Kino T. Tissue glucocorticoid sensitivity: Beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res 2007;39:420-424.
15. Song IH, Gold R, Straub RH, Burmester GR, Buttgereit F. New Glucocorticoids on the Horizon: Repress, Don’t Activate! J Rheumatol 2005;32:1199–1207.
16. Polansky JR, Fauss DJ, Chen P, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 1997;211:126-139.
17. Kwon YH, Fingert JH, Kuehn MH, Alward, WM. Primary open-angle glaucoma. N Engl J Med 2009;360:1113-24.
18. Teklas OY, Hammer, CH, Danias j, et al. Morphologic changes in the outflow pathways of bovine eyes treated with corticosteroids. Invest Ophthalmol Vis Sci 2010;51:4060–4066.
19. Jacobson N, Andrews M, Shepard AR, et al. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet 2001;10:117-25.
20. Aroca-Aguilar JD, Sánchez-Sánchez F, Martínez-Redondo F, Coca-Prados M, Escribano J. Heterozygous expression of myocilin glaucoma mutants increases secretion of the mutant forms and reduces extracellular processed myocilin. Mol Vision 2008;14:2097-2108.
21. Hoare MJ, Grierson I, Brotchie D, et al. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest Ophthalmol Vis Sci 2009;50:1255-63.
22. Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters f-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskel 2005;60:83–95.
23. Job R, Raja V, Grierson I, et al. Cross-linked actin networks (CLANs) are present in lamina cribrosa cells. Br J Ophthalmol 2010;94:1388-1392.
24. Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Safety 2008;31:127-141.
25. Cekic O, Chang S, Tseng JJ, et al. Cataract progression after intravitreal triamcinolone injection. Am J Ophthalmol 2005;139:993-998.
26. Hille B. Ionic channels of excitable membranes. 3rd ed. Sunderland, Mass: Sinauer Associates Inc., 2001.
27. De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharm 2010;10:497–504.
28. Reichardt HM, Kaestner KH, Tuckermann J, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 1998;93:531-41
29. Shacked H, Berger M, Rehwinkel H, Asadullah K. Selective glucocorticoid receptor agonists (SEGRAs): Novel ligands with an improved therapeutic index. Mol Cell Endocrinol 2007;275:109–.
30. Clintrials.gov Dose Escalation of Different Concentrations of ZK 245186 in Atopic Dermatitis and Evaluation of BOL-303242-X Versus Vehicle for the Treatment of Inflammation Following Cataract Surgery, NCT00905450. Accessed 8 February 2011.
31. Zhang JZ, Cavet ME, van der Meid KR, et al. BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells. Mol Vision 2009;15:2606-2616.
32. Pfeffer BA, DeWitt CA, Salvador-Silva M, et al. Reduced myocilin expression in cultured monkey trabecular meshwork cells induced by a selective glucocorticoid receptor agonist: Comparison with steroids. Invest Ophthalmol Vis Sci. 2010;51:437-446.



