More than 16 million American adults have been diagnosed with dry-eye disease, and prevalence increases with age. To help these patients, researchers are looking at new ways to diagnose and treat the condition. Here’s a look at some of the drugs and devices in the pipeline.
Reproxalap 0.25% (Aldeyra Pharmaceuticals)
One promising new product is a small-molecule reactive aldehyde species (RASP) inhibitor that covalently binds free aldehydes and diminishes excessive RASP levels. “Any blockage of RASP could potentially benefit dry-eye patients and allergic conjunctivitis patients,” says Robert Latkany, MD, who is in practice in New York City. “This product is in Phase III trials for both dry eye and allergic conjunctivitis, and the Phase III trial for allergic conjunctivitis showed a reduction of ocular itching score. Additionally, the dry-eye study showed a reduction in symptoms as early as one week.”
RGN-259 0.05% and 0.1% (RegeneRx)
This product is a Tβ4-based sterile and preservative-free eye drop that’s designed to be a novel treatment for dry eye and neurotrophic keratitis. In a Phase II/III study for dry-eye syndrome that included 317 patients, RGN-259 0.05% and 0.1% demonstrated statistically significant improvements in both signs and symptoms of dry eye compared to placebo in a dose-dependent manner during a 28-day dosing period.1
Visomitin (Mitotech)
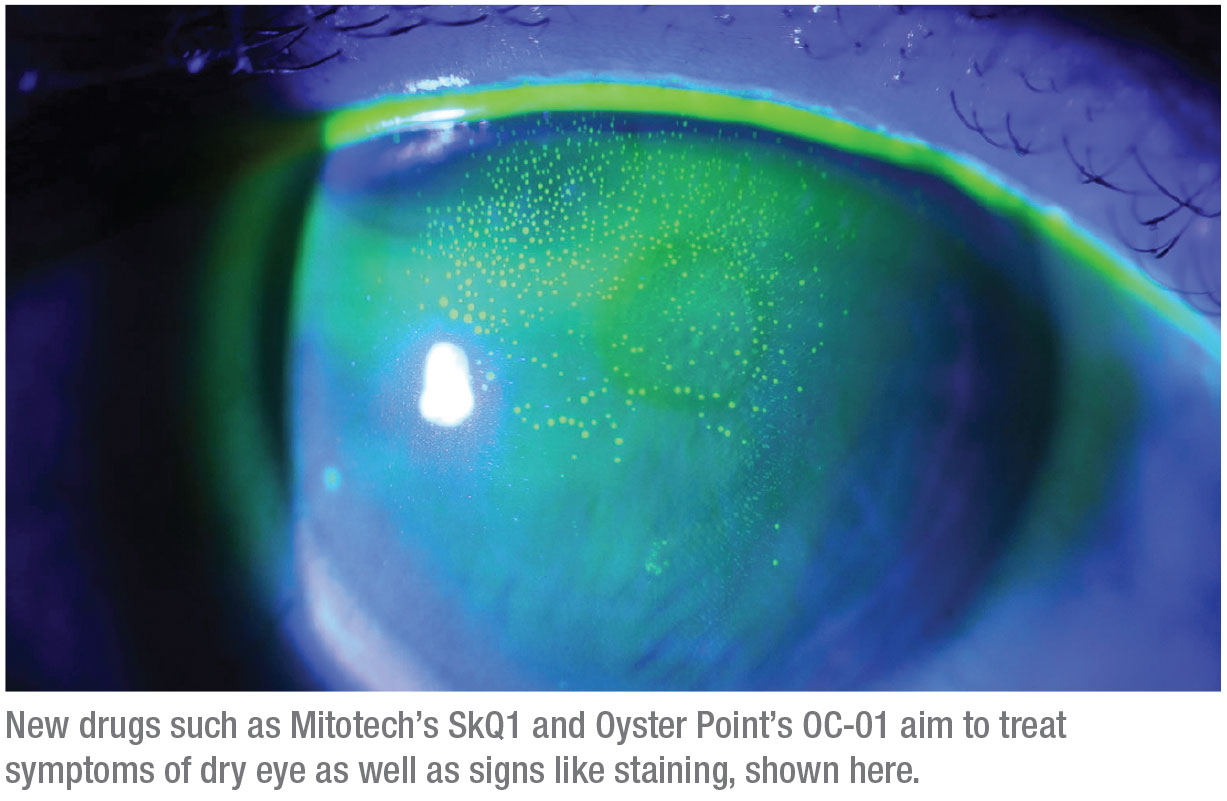
Visomitin is an eye-drop formulation of SkQ1 that’s been developed to target ophthalmic disorders like dry eye, uveitis and macular degeneration. This drop is currently in Phase III trials in the United States.
VISTA-1 was a multicenter, randomized, double-blind, placebo-controlled clinical study involving three treatment arms: two concentrations of SkQ1 and vehicle, administered twice daily.2 The study included approximately 450 patients, who received treatment over a two-month period. While nominal co-primary endpoints of the study (fluorescein staining in central corneal zone and grittiness) weren’t met, multiple predetermined secondary endpoints demonstrated broad action of SkQ1 in the intent-to-treat population. Compared to the vehicle, which was an artificial tear, SkQ1 demonstrated a statistically significant reduction in ocular discomfort (p<0.05) as early as after four weeks of treatment. Additionally, there was a statistically significant improvement in conjunctival fluorescein staining as compared to the vehicle. The drop also had an excellent safety profile, with tolerability being statistically similar to that of an artificial tear.
“The trials were pretty impressive at improving both staining and dry-eye symptoms. I love the approach of going at this from different angles,” Dr. Latkany says.
VISTA-2 is a Phase III study that is similar in design to VISTA-1, but includes two treatment arms (SkQ1 solution and vehicle) with twice as many (300) patients per arm. Enrollment for this study began in December 2019.
 |
OC-01 (Oyster Point Pharmaceuticals)
OC-01 is a nasal spray developed to treat the signs and symptoms of dry eye.
Francis Mah, MD, who is in practice in San Diego, is intrigued by the drug’s mechanism of action. “I think it’s really unique in that you’re potentially stimulating all three sources or layers of the tear film,” he says. “Data show that when mechanically stimulated, in addition to increasing the aqueous layer, the nasal stimulation is increasing the meibum being released from the meibomian glands. Additionally, mucin is being released from the goblet cells in the conjunctiva. I think that’s very unique. If you can stimulate all three layers on demand, I think it’s a very appealing drug for patients and doctors.”
Dr. Latkany says the route of administration may help the drug achieve its effect in unique ways. “This is direct administration of varenicline, which is a nicotinic acetylcholine receptor agonist that might increase tear production,” he says. “It’s clear that you can do things to the nose to reflexively make tears, either through irritation or administering drugs into the nasal cavity. I’m definitely interested in this angle, just because I know reflexively it can make tears and also because some patients have a hard time putting eye drops in their eyes. Looking for alternative ways of administering therapy other than the eyes is always welcome.”
In July 2019, Oyster Point Pharmaceuticals announced the enrollment of the first subject in the Phase III ONSET-2 trial, which is a multicenter, randomized, double-masked, placebo-controlled clinical trial to evaluate the safety and efficacy of OC-01 nasal spray for treating the signs and symptoms of dry eye.3 The study will enroll approximately 750 subjects at approximately 20 centers in the United States and will evaluate two doses (0.6 mg/mL and 1.2 mg/mL) of the spray compared to a placebo nasal spray.
The company says its Phase IIb ONSET-1 study demonstrated statistically significant improvements in both the pre-specified primary sign endpoint and multiple pre-specified secondary symptom endpoints as compared to vehicle. OC-01 was well-tolerated with no significant ocular adverse events or serious drug-related adverse events.
New Cyclosporine Products
There are several new cyclosporine products in various stages of development. According to John Sheppard, MD, who is in practice in Norfolk, Virginia, Novaliq is investigating two products based on the company’s semifluorinated alkane water-free vehicle. “They’ve come up with the first truly new vehicle [EyeSol] for topical eye medications in over a generation,” Dr. Sheppard says. “This semifluorinated alkane permits really small drops. They are 20 µL instead of 50 µL, so there’s no slop or spillover onto your cheek. These drops don’t blur your vision as much either. Furthermore, the vehicle provides a sustained-release platform, homogeneous delivery with favorable first-order pharmacokinetics and distribution of any active pharmaceutical ingredient, including glaucoma medications, for example, as well as anti-inflammatories or immunomodulators. That’s really cool, but what’s cooler is that you can alter the molecular weight of the semifluorinated alkanes. They’ve developed a heavier molecular weight molecule that’s advantageous to meibomian gland function. This product is in Phase III trials, so it should be the first approved in this family of semifluorinated alkanes.”
Additionally, Novaliq has developed a product called CyclASol 0.1%, which is a low molecular weight semifluorinated alkane in a preparation containing cyclosporine. “It produces less stinging and irritation and better distribution of the cyclosporine,” Dr. Sheppard notes. “And, the concentration is 0.1%, so it’s a higher concentration than either Restasis or Cequa. These products will complete their trials in the next year or so, and they should be on the market the year after that.”
In late 2018, Novaliq announced positive topline results from ESSENCE, its first pivotal clinical trial of CyclASol 0.1% for the treatment of dry-eye disease.4
ESSENCE is a pivotal, randomized, double-masked, vehicle-controlled, multicenter Phase IIb clinical trial designed to evaluate the efficacy, safety, and tolerability of topical CyclASol 0.1% for the treatment of patients with aqueous-deficient dry-eye disease. The researchers evaluated the primary efficacy endpoint at four weeks, with continued dosing for efficacy and safety evaluations over a period of three months.
The trial met its primary efficacy endpoint (improvement of total corneal fluorescein staining over vehicle) at Day 29, with high statistical significance (p=0.0002). The effect started as early as two weeks after the initiation of treatment and was maintained for the duration of the study. The central area of the cornea benefited most. The clinical significance of these outcomes is further shown by a high responder rate (>50 percent) on both corneal staining (at four weeks) and conjunctival staining (at three months).
Novaliq’s ESSENCE study also confirmed the excellent safety and tolerability profile of CyclASol. Only 2.5 percent of the CyclASol-treated group ofpatients experienced an instillation site reaction adverse event, the company says.
NOV03 (Bausch Health)
In late December 2019, Bausch Health acquired an exclusive license for the commercialization and development of the investigational treatment NOV03 (perfluorohexyloctane), a first-in-class investigational drug with a novel mechanism of action to treat dry eye associated with meibomian gland dysfunction.5
NOV03 is a proprietary, water-free, preservative-free solution based on Novaliq’s EyeSol technology. In a Phase II study, NOV03 met its primary sign endpoint of improving total corneal fluorescein staining over control at eight weeks, with high statistical significance (p<0.001). It also demonstrated significant and clinically meaningful improvement in a variety of symptoms over the duration of the trial. A Phase III study is already under way, and Bausch Health anticipates starting an additional Phase III study in 2020.
Lacripep (TearSolutions)
Lacripep is a first-in-class topical synthetic peptide treatment for dry eye. It’s a synthetic tear protein fragment of Lacritin, which is a nanomolar concentration constituent of normal human tears, but is lacking in the tears of dry-eye patients. The enrollment for a Phase II trial of Lacripep is complete, and results are expected in the spring of 2020.6 The study, which was conducted at 35 private and academic sites in 17 states, lasted eight weeks and included five visits to an ophthalmologist. Patients were provided with ophthalmic drops of Lacripep or placebo.
“Preclinical studies7 in our laboratory found that both full molecular weight Lacritin and the Lacripep fragment restored tearing and ocular surface integrity,” Dr. Sheppard notes. “It’s an interesting approach. It’s applying a protein that might be deficient and might be needed on the ocular surface.”
Discovery (TearLab)
 |
TearLab is finishing studies for a second-generation machine called Discovery. “Their first machine measures tear osmolarity—Discovery does that and also measures MMP-9,” Dr. Mah explains. “This machine provides increased sensitivity for diagnosing and managing ocular surface disease. Hopes are that it will be approved soon.”
Dr. Sheppard says that the beauty of looking at both osmolarity and MMP-9 is that the osmolarity acts as a normalizing comparator to further refine the accuracy of the MMP-9 test. “So, now you have a screening test for dry eye, and then you can look for the inflammatory component, all in the same tear specimen,” he says. “We’ve used it successfully in our office, and we’re looking forward to approval and distribution in 2020.”
ECF843 by Novartis
Novartis is studying a biologic lubricant found in synovial fluid. ECF843 is a recombinant human lubricin (boundary lubricant) and an investigational compound. Efficacy and safety of ECF843 haven’t been established.8
“The currently available clinical data are impressive. It really seems to be an impressive lubricant and could be a huge game-changer in the way we manage patient complaints of ocular surface disease,” Dr. Mah says. “Having said that, it’ll be interesting to see how the FDA handles this asset. I don’t really know how this is going to be considered—whether the FDA will consider this a drug or a biologic. Novartis hopes to have approval within a year or two.”
LacriPen (LacriScience)
According to Dr. Sheppard, LacriScience has developed a unique technology, the LacriPen, a handheld, portable diagnostic tool that you can carry in your shirt pocket. When touched to a patient’s eye, it allows a specially processed surface plasmon resonance apparatus to come into direct contact with the tear film. The gold tip activator calculates minute changes in the refractive index on an extremely thin gold film. The company says that the device can analyze virtually any component of the tear film based upon its specific resonance profile. The resonance pattern is unique to any number of likely tens of thousands of molecules, LacriScience says. “So, this particular technology can be adapted to osmolarity, to issues with inflammation, to allergy, to infection, and is applicable not only to ophthalmology, but to other bodily fluids and disease states, as well,” Dr. Sheppard explains.
The device currently detects tear osmolarity in the range of 260 to 400 mOsml with a resolution of about 2 mOsml.9 The MMP-9 sensor detects a dynamic range from 1 to 100 ng/mL.
As the preceding list of cutting-edge approaches shows, our understanding of dry eye and its mechanisms is evolving, and so are our ways of treating the condition. REVIEW
Dr. Sheppard has a financial interest in LacriScience and serves as a consultant to Allergan, Sun Pharma, Novaliq, Aldeyra, Tear Science, Oyster Point, Novartis, Bausch & Lomb and TearLab. Dr. Mah has a financial interest in Allergan, Bausch & Lomb, Novartis, Senju, Sun and TearLab. Dr. Latkany has no financial interest in any of the products or companies mentioned in this article.
1. RegenRx. regenerx.com/RGN-259
2. News release. www.mitotechpharma.com/news/mitotech-and-essex-bio-technology-announce-enrollment-in-vista-2-a-pivotal-phase-3-clinical-study-of-skq1-for-dry-eye-disease
3. News release. https://oysterpointrx.com/oyster-point-pharma-announces-enrollment-of-first-subject-in-phase-3-clinical-trial-of-nasal-spray-for-dry-eye-disease/
4. News release. https://www.businesswire.com/news/home/20181017005727/en/Novaliq-Announces-Positive-Topline-Results-CyclASol%C2%AE-Phase
5. News release. https://www.prnewswire.com/news-releases/bausch-health-licenses-novaliqs-nov03-investigational-treatment-for-dry-eye-disease-associated-with-meibomian-gland-dysfunction-300978312.html
6. Data on file. https://tearsolutions.com/clinical-trial-sites/
7. Samudre S, Lattanzio FA Jr, Lossen V, et al. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Investigative Ophthalmology & Visual Science 2011;52:6265-6270.
8. News Release. https://medinfo.novartispharmaceuticals.com/servlet/servlet.FileDownload?file=00P1H00001GiZaeUAF&sfdcIFrameOrigin=null
9. Company background. https://ois.net/lacriscience/



