Positive Dysphotopsia
There are two types of
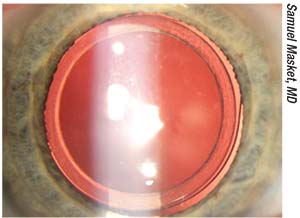 |
| Figure 1. First version of the Morcher 90S anti-dysphotopic IOL. The anterior capsulotomy fits into a peripheral groove in the IOL. Note the excellent centration. |
Positive dysphotopsia is characterized by undesired light streaks, arcs, and flashes that emanate from obliquely incident sources of light. “The literature is clear that the chief cause of positive dysphotopsia is square-edge IOLs, which became popular in the mid-90s because of their ability to reduce the incidence or retard the development of posterior capsule opacification. We really didn’t notice positive dysphotopsia until the advent of square-edge IOLs,” says Samuel Masket, MD, who is in practice in Los Angeles.
He notes that another, less widely known cause of positive dysphotopsia is reflections from the internal back surface of the front of the IOL. This observation1 is typically associated with high-index-of-refraction, low-radius-of-curvature IOLs. “The manufacturing sector eventually changed IOL design, putting more of the power on the front of the lens, which helped reduce internal reflection. Additionally, manufacturers altered the square edge of the IOL by making the front edge round or frosting the square edge. However, because positive dysphotopsia is due to internal reflection, altering the external edge of the lens seems unlikely to help,” Dr. Masket explains.
According to Dr. Masket, all IOLs on the U.S. market today have square edges. He notes that because the index of refraction and the surface reflectivity of lenses can be associated with positive dysphotopsia, certain IOLs will show less of a tendency. “For example, silicone and copolymer lenses tend to have a much lower incidence of positive dysphotopsia than acrylic lenses, but any truncated-edge IOL, including oval lenses,2 will be associated with positive dysphotopsia. So, it still exists, and my sense is that the manufacturing sector has paid closer attention to multifocality, asphericity and toricity of IOLs than to patient-generated complaints,” he says.
Unfortunately,
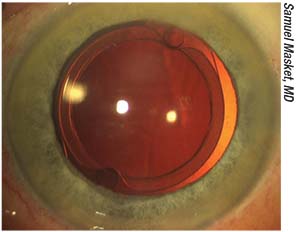 |
| Figure 2. A modified Morcher 90S IOL. Note the peripheral groove and excellent centration. Fixation holes facilitate capture of the IOL within the anterior capsulotomy. |
Negative Dysphotopsia
According to Dr. Holladay, negative dysphotopsia became more common in the 1990s for three reasons: square optics were introduced, acrylic lenses (which have a higher index of refraction) were introduced, and surgeons began leaving an overlap of about 0.5 mm of the anterior capsule over the anterior edge of the optic of the lens.
“These were all improvements in IOLs that reduced posterior capsular opacification, but there was a trade-off. We increased the incidence of negative dysphotopsia in exchange for reducing posterior capsular opacification,” he says.
Negative dysphotopsia is an observation by a patient of a phenomenon similar to a horse blinder. There is a dark arc on the temporal side of the vision. “What causes it remains controversial today. Because of that controversy, it hasn’t really been well approached by the manufacturing sector. Ray-tracing theoretical studies, particularly two studies from Jack Holladay, have indicated that IOL design, material, position and other factors could be causal. However, some of those parameters haven’t matched the clinical picture,” Dr. Masket adds.
According to one of Dr. Holladay’s studies published earlier this year,3 standard ray-tracing techniques showed that a shadow is present when there’s a gap between the retinal images formed by rays missing the optic of the IOL and rays refracted by the IOL. Additionally, the ray tracing also showed a constriction and double retinal imaging in the extreme temporal visual field. This study found that, in a nominal acrylic pseudophakic eye model with a 2.5-mm diameter pupil, the maximum retinal field angle from rays refracted by the IOL was 85.7 degrees, and the minimum retinal field angle for rays missing the optic of the IOL was 88.3 degrees, which left a dark gap of 2.6 degrees in the extreme temporal field. “We also showed that there are patient factors that increase the risk of this, such as an extremely small pupil, a high intraocular lens power and a larger angle kappa,” Dr. Holladay explains. “Angle kappa is the angle between the visual axis and the pupillary center. The bigger that is, or the more central your pupil is in the cornea away from the visual axis, the higher the chance of negative dysphotopsia.” Secondary factors included the following: IOL edge design; material; diameter; decentration; tilt; and aspheric surfaces.
This study3 explains in detail all of the factors associated with negative dysphotopsia, says Dr. Holladay. “In the simplest of terms, there is a gap between the rays refracted by the IOL and those missing the IOL, which leaves a crescent-shaped shadow on the retina. I don’t believe anyone who has read this article still believes there is controversy as to the cause,” he says.
Interestingly, and frustratingly, for the surgeon and patient, negative dysphotopsia only occurs after perfect surgery. “The posterior capsule is perfectly clean, the capsulorhexis perfectly overlaps the optic, and the lens is perfectly centered. It’s like the perfect cases are the ones who are most likely to experience it,” says Kevin M. Miller, MD, who is in practice in Los Angeles.
Dr. Masket agrees that these patients have a perfectly centered IOL underneath the continuous anterior capsulotomy. “Negative dysphotopsia occurs in as many as 15 percent of patients early after surgery, although the great majority improve over time, bringing the incidence down to about 3 percent at one year.4 We’ve just completed a clinical study5,6 that parallels our original work that indicates that negative dysphotopsia can be associated with any of the intraocular lenses that are on the market in the United States, as long as that lens is well-centered in the capsular bag,” Dr. Masket says.
Symptoms will resolve with reverse optic capture, meaning that the optic portion of the lens is moved in front of the capsular bag into the ciliary sulcus, leaving the loops in the capsular bag. “There seems to be some relationship with the lens and the capsular bag, and that is the final common pathway,” Dr. Masket adds, “although the cause of negative dysphotopsia is likely multifactorial and still somewhat enigmatic. For example, in our study, we found a preponderance of negative dysphotopsia in women and in left eyes. Those phenomena are mysterious to us.”
He notes that making the pupil smaller, which helps positive dysphotopsia, actually makes negative dysphotopsia worse. “Dilating the pupil makes the symptoms better, but, unfortunately, dilation can induce glare and nighttime difficulties. So, pupil dilation is not an appropriate strategy for these patients,” Dr. Masket says.
Patients can be offered spectacles that block the light coming from the side that is stimulating negative dysphotopsia. If the symptoms improve over time or the patient doesn’t mind wearing glasses, then surgery doesn’t need to be considered. “As far as surgical treatment, we strongly prefer reverse optic capture. In the case of a single-piece IOL, the haptics must remain in the bag, so we manipulate the capsulotomy to go under the edge of the optic rather than have it overlay the optic. This strategy requires that the anterior capsulotomy be the right size and well-centered. If it isn’t, then we encourage lens exchange with a three-piece lens placed in the ciliary sulcus, and we suture-fixate the loops so the iris of the lens doesn’t decenter. Reverse optic capture has eliminated or significantly improved the symptoms in nearly all of our patients. We’ve also used reverse optic capture for the second eye of patients who are very symptomatic in their first eye after surgery. We have employed this strategy for 21 cases, and none of those second eyes have had negative dysphotopsia,”7 Dr. Masket says.
For patients who have combined negative and positive dysphotopsia, he recommends exchanging the lens for a three-piece copolymer or three-piece silicone lens with reverse optic capture. “We’ve had excellent success with that technique,” he notes.
Dr. Masket has designed an IOL specifically to prevent negative dysphotopsia. The design attempts to mimic the optical advantage of reverse optic capture while leaving the bulk of the IOL within the capsule bag. “The anti-dysphotopic IOL is manufactured in Germany by Morcher, and it has been used in limited clinical trials and modified as appropriate over time. Presently, 100 of the IOLs have been implanted, and none of those patients have had negative dysphotopsia at any time, providing proof of concept,” he adds.
According to Dr. Miller, in the past, the best solution for patients with negative
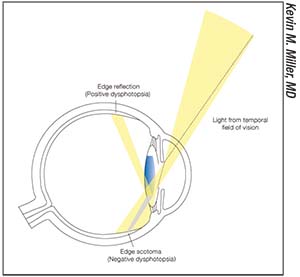 |
| Figure 3. Light entering an eye from the temporal field of vision crosses the pupil and encounters the flat edge of a high-index-of-refraction intraocular lens. Some of the light bounces off the edge, creating one of the positive dysphotopsias. Distal to the edge, a shadow is cast onto the nasal retina, creating a negative dysphotopsia. |
Another treatment option is to orient the optic-haptic junction of an acrylic lens at 3 o’clock and 9 o’clock, so that the optic-haptic junction is horizontal. “The amount of negative dysphotopsia that patients experience goes from 25 or 30 percent down to 5 percent. The improvement is dramatic,” he explains.
A recent study conducted by Bonnie Henderson, MD, and colleagues found that positioning the optic-haptic junction of an acrylic IOL inferotemporally resulted in a 2.3-fold decrease in the incidence of negative dysphotopsia after cataract surgery.7 When implanted in the vertical position, acrylic IOLs seem to have a higher incidence of negative dysphotopsia than silicone IOLs.
In this study, patients had surgery with implantation of either a silicone IOL inferotemporally or vertically or a one-piece acrylic IOL with the optic-haptic junction inferotemporally or vertically. Other patients received acrylic IOLs bilaterally and inferotemporally without randomization. Patients were asked about negative dysphotopsia symptoms postoperatively.
The study included 418 eyes in 305 patients. A silicone IOL was implanted inferotemporally in 39 eyes and vertically in 60 eyes. An acrylic IOL was implanted with the optic-haptic junction inferotemporally in 163 eyes and with the junction vertical in 114 eyes. Forty-two eyes had bilateral inferotemporal implantation of an acrylic IOL. For the acrylic IOL on the first postoperative day, the incidence of negative dysphotopsia was 6 percent for the inferotemporal IOL orientation, compared with 14 percent for the control group. The rate of persistent negative dysphotopsia decreased in both groups over time, and the difference was no longer statistically significant at one month postop. No negative dysphotopsia was observed with the silicone IOL.
The Future
According to Dr. Masket, not much attention has been paid to patient-reported outcomes with regard to dysphotopsia, despite the fact that dysphotopsia is the leading cause of patient dissatisfaction after otherwise uncomplicated cataract surgery.8 However, that is changing. “There is now a group effort by the American Academy of Ophthalmology, the FDA and the manufacturing sector to develop a patient questionnaire to determine how patients perceive visual function with premium IOLs. Given that dysphotopsia, both negative and positive, has been problematic, it is likely that more attention will be directed to reducing these undesirable optical phenomena,” he adds.
Dr. Masket holds patents for IOL design and has a financial interest in the Morcher 90S IOL. Drs. Miller and Holladay don’t have a financial interest in any product mentioned in the article.
1. Erie JC, Bandhauer MH, McLaren JW. Analysis of postoperative glare and intraocular lens design. J Cataract Refract Surg 2001;27:4:614-621.
2. Masket S, Geraghty E, Crandall AS, et al. Undesired light images associated with ovoid intraocular lenses. J Cataract Refract Surg 1993;19:6:690-694.
3. Holladay JT, Simpson MJ. Negative dysphotopsia: Causes and rationale for prevention and treatment. J Cataract Refract Surg 2017;43:2:263-275.
4. Osher RH. Negative dysphotopsia: long-term study and possible explanation for transient symptoms. J Cataract Refract Surg 2008;34:10:1699-1707.
5. Masket S, Fram N. Pseudophakic negative dysphotopsia: Surgical management and new theory of etiology. J Cataract Refract Surg 2011;37:7:1199-1207.
6. Masket S, Fram N, et al. Surgical management of negative dysphotopsia. J Cataract Refractive Surg. In review.
7. Henderson BA, Yi DH, Constantine JB, Geneva II. New preventative approach for negative dysphotopsia. J Cataract Refract Surg 2016;42:10:1449-1455.
8. Tester R, Pace NL, Samore M, Olson RJ. Dysphotopsia in phakic and pseudophakic patients: Incidence and relation to intraocular lens type (2). J Cataract Refract Surg. 2000;26:6:810-816.



