Many physicians managing glaucoma patients have accepted the idea that macular damage only occurs late in the disease. In recent years, however, a number of studies have provided a growing body of evidence that macular damage occurs in glaucoma patients earlier and more frequently than previously believed. Meanwhile, our understanding of how vision is impaired by the disease—especially in early glaucoma—has been lacking. In the clinic, I find it frustrating that what I see in the 24-2 visual field data often doesn’t correlate with what the patient is reporting.
Of course, a correlation between visual complaints and visual field data is easy to find when glaucoma is advanced. However, the traditional assumption has been that early glaucomatous damage is primarily peripheral. For that reason, a glaucoma patient’s central vision complaints have usually been attributed to other possible factors such as co-existing pathologies. Maybe glaucoma eye drops are causing corneal dryness that could explain visual glare complaints. Maybe the patient has early macular degeneration. Maybe the drops are speeding the development of cataract. Maybe peripheral visual field loss is responsible in some way. Maybe the complaints can be attributed to the way binocular vision combines slightly different images.
With evidence now suggesting that central macular damage might be occurring much sooner than previously thought, I saw a potential explanation for these difficult-to-explain central-vision complaints. So, our group decided to explore the relationship between glaucomatous macular damage and the patient’s everyday visual function—especially in early disease.
Here, I’d like to share some of what we’ve discovered about the presence of macular damage in early glaucoma; how we can miss that damage when we rely exclusively on the 24-2 visual field test; and how that damage may be tied to patient visual complaints that were previously attributed to those other hypothetical causes.
Central Vision Complaints
First of all, it’s important to understand that many vision com-plaints coming from patients with early glaucoma are, in fact, central vision complaints. One 2013 study asked 50 glaucoma patients with mild to moderate disease, visual acuity better than 20/30 and a range of visual field defects in both eyes, to identify one of six Photoshopped images that best approximated their perceptions of their vision difficulties.1 The six options were: a tunnel of clear vision surrounded by darkness; a tunnel of clear vision surrounded by blur; clear vision with dark patches sprinkled across the visual field; clear vision with blurred patches; clear vision with areas of missing information; and no awareness of altered vision.
Based on traditional ideas about peripheral damage in early glaucoma, we’d expect patients to select the clear vision surrounded by either blur or darkness. Instead, no patients chose the clear tunnel surrounded by darkness and only two patients (4 percent) chose the clear tunnel surrounded by blurriness. No one selected the image with dark patches, but 54 percent chose the image with blurred patches, and 16 percent chose the image with areas of missing information. (26 percent reported being unaware of any vision loss.) In essence, three-quarters of the patients reported visual symptoms that were more central than peripheral.
As a follow-up, the authors examined the relationship between the presence of visual symptoms and the 24-2 visual field. They couldn’t find a clear linear relationship between 24-2 mean deviation and visual symptoms. This supports the clinical observation that visual complaints correlate poorly with 24-2 visual field results in early glaucoma patients.
Another study asked 99 patients with different types and stages of glaucoma (76 percent had primary open-angle glaucoma) to report which of a list of visual complaints they were experiencing.2 The authors found that the three most common complaints from glaucoma patients were all central vision issues—specifically, needing more light to see well, blurred vision and glare. Peripheral complaints (e.g., difficulty seeing objects off to either side) were much less frequently reported.
Lastly, a third, case-controlled study questioned 221 glaucoma patients about their visual performance under different luminance conditions.3 Glaucoma patients were found to have significantly more complaints in glare or low-luminance conditions than controls. Circumstances reported to be problematic included driving on a cloudy day, seeing outside at night when there’s no moonlight, reading in the sun and adapting to bright or dim light. The idea that glaucoma patients not only have complaints about central vision but also have trouble adapting to light or dark conditions is surprising; these are complaints we normally associate with photoreceptor damage or loss. (In fact, some research relating to macular degeneration is suggesting that difficulty with dark adaptation could be a very early sign of macular degeneration.)
These findings make it clear that the relationship between the 24-2 visual field—the classic glaucoma functional test—and everyday visual performance in glaucoma patients is not well-understood. Clearly, the relationship is not as straightforward as was previously believed.
Pursuing a Macular Mystery
To delve into this issue, we decided to conduct a series of studies to address the different questions raised by the possible connection between vision complaints from patients with early glaucoma and macular damage.
In our first study we explored whether glaucoma patients with macular damage had worse vision-related quality of life.4 Patients had early to moderate glaucoma with no evidence of coexisting dry-eye dis-ease or visually significant cataract, based on the LOCS III system. We used patients’ 24-2 visual field, which is more of a classic peripheral glaucomatous visual field damage measurement, and the 10-2 visual field, which measures central or macular functional loss. To evaluate patients’ quality of life, we had them fill out the National Eye Institute Visual Function Questionnaire, a well-validated measure of vision-related quality of life that not only measures patients’ perceptions of their visual loss, but also how it impacts their day-to-day functioning.
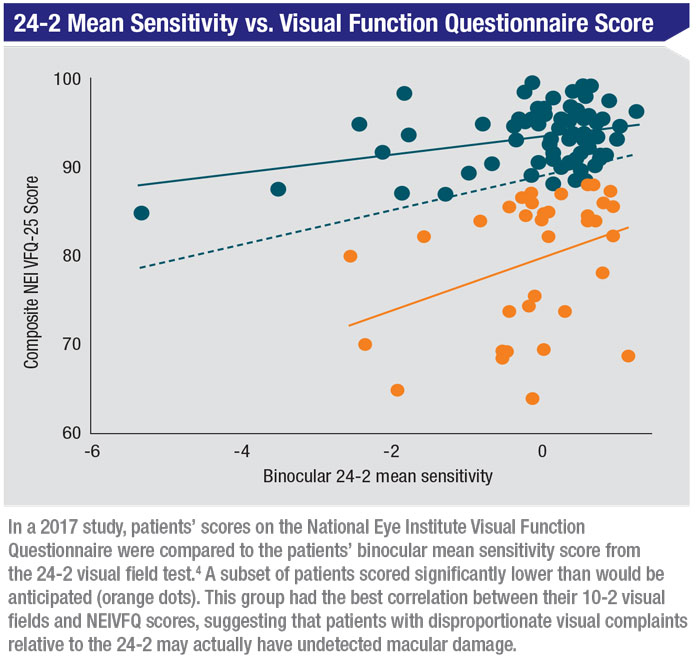 |
Not surprisingly, we found that the 10-2 visual field is a better predictor of the NEIVFQ score than the 24-2 visual field. But the most interesting data appeared when we plotted the binocular 24-2 mean sensitivity, which integrates the two separate eyes into a single score, relative to the NEIVFQ. (See chart, above.) A subset of patients had NEIVFQ scores significantly lower than would be anticipated based on the 24-2 visual fields. This group turned out to have the best correlation between their 10-2 visual fields, focused on the macular region, and their NEIVFQ scores. We concluded that patients with disproportionate visual complaints relative to the 24-2 may actually have undetected macular damage.
Our next study sought to investigate whether macular damage was impacting vision-related quality of life across the different stages of glaucoma.5 This is a pretty intuitive conclusion to reach in patients with later-stage disease, but we wanted to see if there was a correlation in patients with early disease, whom we haven’t traditionally thought of as having macular damage.
To investigate this we looked at 88 eyes of 44 patients with early open-angle glaucoma (defined as a 24-2 mean deviation better than -6 dB). Subjects could not have macular de-generation, had to have less than 2+ nuclear cataract using the LOCS III grading system and minimal to no PCO. We identified the presence of any macular damage in the better and worse eyes, as well as what I’ll refer to as peripheral arcuate damage—glaucomatous damage outside of the macula. Then, we looked to see which patients had NEIVFQ scores that were more than one standard deviation below the mean. We found:
- Forty-four of the worse eyes (57 percent) had macular damage; 13 of the better eyes (31 percent) had macular damage.
- Patients with confirmed macular damage in the better eye were 35 times more likely to be in the low-quality-of-life score group.
- Patients with macular damage in the worse eye were 11 times more likely to be in that group.
- Perhaps most interesting, peripheral damage was not an independent predictor for being in the low-quality-of-life-score group.
- Our conclusions were first, that mac-ular damage is common in early glaucoma; and second, even in early glaucoma, macular damage can impact vision-related quality of life, while peripheral damage does not.
Diffuse vs. Focal Damage
Next we decided to look at whether the pattern of macular damage affected the NEIVFQ score. Two types of macular damage have been described in the existing literature: focal damage, which we can readily see, and diffuse damage. Typically, focal damage is a dense, fairly deep loss of retinal ganglion cells, usually in the inferotemporal macula. This corresponds to a classic superior paracentral visual field defect. Such damage is frequently seen, for example, in normal-tension glaucoma.

|
Diffuse macular damage—a subtle, diffuse, generalized loss of RGCs in the macula—can be much more difficult to identify. It corresponds to a depressed total mean deviation in the 10-2 visual field. This kind of diffuse damage would be especially difficult to identify without macular OCT; we’d probably look at the 10-2 and conclude that this depression was caused by cataract or dry eye.
To address this, we conducted a cross-sectional prospective study involving 214 eyes of 107 patients representing a wide range of glaucomatous damage.6 All eyes under-went 10-2 visual field tests and SD-OCT scans measuring both macular damage and the thickness of the RGC plus IPL layers. (We included the thickness measurement because patients with diffuse dam-age have thinner maculas on average. We wanted to be able to correct for that factor to make sure the thick-ness alone couldn’t account for any differences that turned up be-tween focal and diffuse damage.) All participants also completed the NEIVFQ questionnaire.
The data showed that patients with diffuse damage had significantly lower quality-of-life scores than patients with focal loss (p=0.03), even when adjusted for the average RGC thickness (p=0.02). This tells us that it’s not just a matter of the degree of damage, but the type of damage; patients with focal damage had a more recognizable defect, but more diffuse damage had a greater impact on central vision. A possible explanation for this could be that our brain is able to compensate for a focal defect, just as it does for our natural blind spot, whereas diffuse damage might be nearly impossible to compensate for. (A paracentral defect could certainly affect a patient’s quality of life, but it might not do so to the same degree as more global, diffuse damage.)
This raises an interesting question: Can diffuse macular damage be seen in 24-2 results (beyond a depressed mean deviation score)? Possibly—but macular damage is very easy to overlook in 24-2 results. Any macular damage would be contained within the four central points, so you might only see one or two depressed points. That might fail to draw your attention.
Our next study evaluated the pre-sence and type of macular damage that was associated with visual problems under either low luminance or glare.7 As noted earlier, other studies have found that patients with glaucoma reported having visual problems un-der suboptimal lighting conditions. We decided to see whether macular damage could be a potential driver of these visual complaints.
We conducted an observational cohort study involving 252 eyes of 126 participants who had mild or moderate open-angle glaucoma, examining the relationship between glaucomatous macular damage and visual difficulty under low luminance conditions. This was measured using the Low Luminance Questionnaire, a validated questionnaire mostly used in the retinal literature to look at macular degeneration symptoms. Subjects could not have macular degeneration; they had to have less than 2+ nuclear cataract using the LOCS III grading system; and minimal to no PCO. As before, we divided the eyes into those with diffuse macular damage and those with focal macular damage. Focal and diffuse macular defects were identified using SD-OCT and both 24-2 and 10-2 visual fields. Findings included:
- Sixty-five percent (n=82) of the 126 better eyes showed evidence of macular damage; 35 percent (n=44) of these eyes did not.
- Of the 82 eyes with damage, 40 percent had diffuse damage and 60 percent had focal damage.
- Diffuse macular damage was a significant predictor of having difficulty in extreme lighting (p=0.0024); difficulty in low lighting (p=0.037); and diminished mobility (p=0.042).
- There was no significant difference between subjects who had focal damage and subjects with no macular damage at all.
Real-world Consequences
Lastly, we looked at a real-world functional outcome by examining whether macular damage would impair facial recognition in patients with good central visual acuity. We conducted a prospective, cross-sectional study involving 144 eyes of 72 participants with a diagnosis of open angle glaucoma in one or both eyes and a visual acuity of 20/40 or better in each eye.8 We used SS-OCT and 10-2 visual field testing to determine the presence or absence of macular damage and then tested patients using the Cambridge Face Memory Test, a well-validated test from the neurologic literature. The study had very strict inclusion/exclusion criteria; participants had to have good cognitive function, and patients with miotic pupils, cataracts, surface disease, retinal disease or drusen (among other factors) were excluded.
We found that the presence of macular damage predicted diminished facial recognition, regardless of whether we tested the patient’s better or worse eye (p<0.0001 in either eye). This was true even after adjusting for potential confounding factors such as glaucoma severity, contrast sensitivity, age and visual acuity.
Since then, we’ve conducted another study looking at the correlation between facial recognition ability and diffuse versus focal macular damage.9 The data showed that patients with diffuse damage did significantly worse on the facial recognition test than those with focal damage. Patients with diffuse damage in the better eye recognized 10 fewer faces than those with focal damage; patients with diffuse macular damage in the worse eye recognized an average of 5.5 fewer faces. This remained significant after adjusting for possible confounding factors such as 24-2 mean deviation, age, visual acuity, presence of early cataract, number of drops and contrast sensitivity.
To sum up, our studies to date have demonstrated that:
1) vision-related quality of life, as measured by the NEIVFQ, appears to be driven by diffuse macular damage rather than focal loss;
2) even subtle, diffuse macular damage, easily overlooked in a 24-2 visual field, can cause visual disability;
3) visual complaints relating to low luminance, dim lighting and glare are all significantly associated with diffuse macular damage;
4) facial recognition is affected by the presence of diffuse macular damage.
We believe this provides strong support for the idea that early identification of the presence and type of macular damage is critical to assessing and treating visual disability in early glaucoma. To really assess the patient’s visual performance ac-ross the disease spectrum, we need to look at the correlation between macular OCT and 10-2 visual fields. Early identification of any type of macular damage, focal or diffuse, is important—but it appears to be particularly important to identify diffuse, generalized macular damage.
Practical Pearls
How might a clinician use this information? Here are a few suggestions:
• Run a baseline OCT macular scan on every glaucoma patient. I look for any abnormalities on the macular scan and in the central points of the 24-2 visual field. If I see evidence of damage in either one, I’ll run a 10-2 test.
• Treat early glaucoma patients with macular damage more aggressively. Once I’ve uncovered macular damage, I keep a closer watch on the patient and run 10-2 visual fields more frequently.
• Let the patient know that any central vision issues they’re having may be partly due to the glaucoma. Noting that central visual problems can be connected to their glaucoma will reassure the patient that A) the patient isn’t imagining these problems; B) you understand what the patient is going through; and C) it’s really important for the patient to follow the treatment plan.
• If your patient has central vision symptoms that may be the result of macular damage, consider removing a cataract earlier than you otherwise would have. You can explain to the patient that you can’t correct the macular damage, but you can eliminate the early cataract that’s making the problem worse, instead of waiting for the cataract to become more problematic.
Moving Forward
Being able to better understand your patient’s visual ability in early glaucoma using office-based testing is a real step forward in ophthalmic care. Once significant damage has been done, we can look at the visual field and say it’s obvious that this patient is going to function poorly. But in the early stages of glaucoma, that’s been difficult or impossible to do. (This stands in contrast to many retinal diseases where there’s a linear relationship between central visual acuity and visual function.)
As doctors, it’s hard for us to change our paradigms. We’re taught to be careful and to question change, because we don’t want to adopt anything unproven that might put our patients at risk. But now, thanks in part to advanced technologies such as OCT, we’re getting new insights about diseases like glaucoma, allowing us to look at them and manage them in a more nuanced way. We shouldn’t hesitate to use those new insights to help our patients. REVIEW
Dr. Blumberg is an associate professor of ophthalmic sciences at Columbia University Medical Center, part of the Edward S. Harkness Eye Institute in New York. She reports no financial ties relevant to any products mentioned in this article.
1. Crabb DP, Smith ND, Glen FC, et al. How does glaucoma look? Patient perception of visual field loss. Ophthalmology 2013;120:6:1120-6.
2. Hu CX, Zangalli C, Hsieh M, et al. What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. Am J Med Sci 2014;348:5:403-9.
3. Bierings RAJM, van Sonderen FLP, Jansonius NM. Visual complaints of patients with glaucoma and controls under optimal and extreme luminance conditions. Acta Ophthalmol 2018;96:3:288-294.
4. Blumberg DM, De Moraes CG, Prager AJ, et al. Association between undetected 10-2 visual field damage and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol 2017;135:7:742-747.
5. Garg A, Hood DC, Pensec N, et al. Macular damage, as determined by structure-function staging, is associated with worse vision-related quality of life in early glaucoma. Am J Ophthalmol 2018;194:88-94.
6. Prager AJ, Hood DC, Liebmann JM, et al. Association of glaucoma-related, optical coherence tomography-measured macular damage with vision-related quality of life. JAMA Ophthalmol 2017;135:7:783-788.
7. Blumberg DM, Liebmann JM, Hirji SH, Hood DC. Diffuse macular damage in mild to moderate glaucoma is associated with decreased visual function scores under low luminance conditions. Am J Ophthalmol 2019;208:415-420.
8. Hirji SH, Liebmann JM, Hood DC, et al. Macular damage in glaucoma is associated with deficits in facial recognition. Am J Ophthalmol 2020;217:1-9.
9. Hirji SH, Hood DC, Leibmann JM, Blumberg DM. Association of patterns of glaucomatous macular damage with contrast sensitivity and facial recognition in patients with glaucoma. JAMA Ophthalmol. Published online November 5, 2020. doi:10.1001/jamaophthalmol.2020.4749.