Mark B. Abelson, MD, Andrew Workman, and Ava Taylor, North Andover, Mass.
For decades, we've known that bacteria play a role in instigating blepharitis, but we haven't been able to identify a species on the blepharitic eyelid that's consistently at fault. It turns out that, just as in high school, a little gossip can cause a lot of problems. Microbiologists have discovered that chatter among all bacterial species, a process known as quorum sensing, communicates population sizes. As a result of this chatter, various pathways can form, including the production of virulence factors by commensal bacteria. Eavesdropping on these bacterial conversations promises to vastly improve our understanding of the disease. Here's what we know about blepharitis, and some of the latest findings in the intriguing field of quorum-sensing research.
The State of
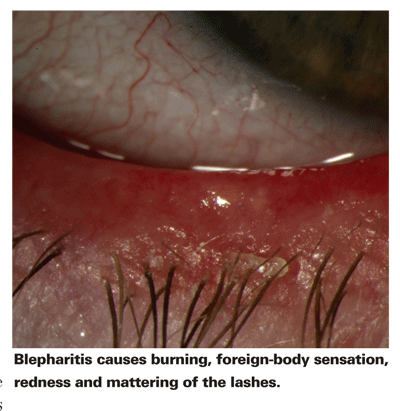
Blepharitis is a common, pervasive, chronic ocular inflammatory disease. It is also characterized by flare-ups and remissions, which have complicated our understanding of its etiology. Its hallmark symptoms are thickening of the eyelid, eyelid burning, crusting and redness of the eyelid margin, abnormal meibomian gland secretions, meibomian gland obstruction and bacterial infection.
Although bacteria are known to be involved in the pathogenesis of blepharitis, consistent evidence of virulent bacterial presence has been difficult to quantify. The discovery of bacterial quorum sensing has led us to the realization that blepharitis is more complicated than originally thought, and likely involves not a single bacterial infection, but rather an imbalance of multiple bacterial species. These species, when properly balanced, may not always be pathogenic.
Standard treatments for blepharitis are simplistic and archaic, and fail to target and treat the underlying causes of the disease. In a testament to the complexity of blepharitis, the 2003 Preferred Practice Pattern, published by the
Categorizing
For decades, blepharitis research has focused on attempting to categorize, describe and explain the disease, as well as suggest treatments. In 1982, James McCulley, MD, classified chronic blepharoconjunctivitis into six categories.1 Unfortunately, the symptoms and causes of blepharitis often overlap, complicating diagnosis and treatment. Fungi, for example, can become involved: Pityrosporum ovale, which derives nutrients from fat, is found in areas with many sebaceous glands. It appears to be involved in the pathology of seborrheic dermatitis. Tiny arthropods have also been identified as blepharitic instigators. Two species of Demodex mites are known to live in human eyelash follicles, and although they are normally benign, can burgeon in population in response to depressed immune systems or advanced age. Their eggs and decomposing bodies then clog the meibomian glands, resulting in blepharitis. Finally, angular blepharitis involves the blocking of the lacrimal ducts, and may be caused by staphylococcal (gram-positive) and/or moraxella (gram-negative) bacteria.
Hide-and-Seek Bacteria
The bacterial component of blepharitis has long been suspected,2 but a precise description of its nature is only starting to come into view. We know that Staphylococcus aureus and Staphylococcus epidermidis, along with other flora (Table 1), are characteristic bacterial inhabitants of both healthy human eyelids and blepharitic eyelids, but exist at a higher level on blepharitic eyelids.3,4 Why then are some eyelids diseased and some not?
Bacteria of the eyelid are unpredictable, and they appear, disappear and reappear over time.5 They can alter their population levels in response to a number of factors, including the components of tear-film residue, available space, available nutrients, host environment/geographic location and host immune response. Quorum sensing is the ability of bacteria to react to their own population density, and is governed by bacterial secretion of molecules called auto-inducers.
As a bacterial population grows, the level of auto-inducers rises, eventually reaching a threshold for the activation of signaling pathways in bacteria. These signaling pathways result in the expression of many different factors, including those associated with virulence.6 Once they have sensed a particular population size, their genes trigger them into action, resulting in anything from bioluminescence to biofilm formation—or even the production of virulence factors.
Polymicrobial Blepharitis
The quorum-sensing field is 10 years old, but evidence of bacterial communication emerged in the 1960s and '70s. Among the first evidence was Harvard researcher J. Woodland Hastings' observation that bioluminescent marine bacteria emitted light at high, but not low, cell density.7 This was little more than an interesting observation until we turned in our rabbit-ear antennae and 8-tracks for cable TV and CDs. Then, in the early '90s, the quorum-sensing field was born of many talented researchers, most notably Princeton's Bonnie Bassler, PhD, and 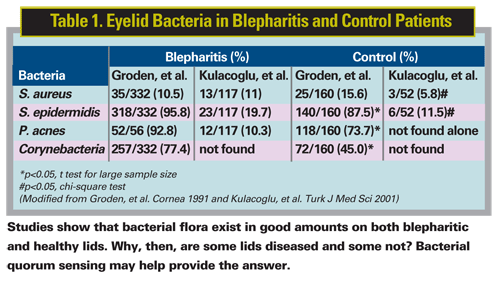
Flora populations, in any healthy part of the body, reside in levels conducive to a symbiotic relationship with the host. Bacteria on healthy skin may remain non-pathogenic until their population increases beyond a point of critical mass, disrupting their symbiotic relationship with the host. Furthermore, these bacteria exist a priori, making it difficult to decipher friend from foe. Indeed, bacteria that are benign one day may become virulent the next. Strains of S. epidermidis, one of the bacteria most often isolated from the eyelids of blepharitis patients, can produce virulence factors known as phenol-soluble modulins as a result of quorum-sensing gene activation. These PSMs are pro-inflammatory mediators,4 and are produced by S. epidermidis in the late stage of infection when their density has increased, leading to the production of inflammatory cytokines and the attraction of neutrophils.5,6 S. aureus virulence factors have also been shown to attract neutrophils,7 and both S. aureus and S. epidermidis produce proteases that have been shown to induce CXC chemokines in vitro, a role that is hypothesized to be at the root of neutrophilic inflammation in chronic rhinosinusitis.8 It may be hypothesized that the levels of S. epidermidis and S. aureus on the eyelids of healthy individuals are below the population density necessary for quorum-sensing gene activation, while the bacteria on the blepharitic eyelid have overgrown to the point of critical mass. Although S. aureus and S. epidermidis are often branded as the bad guys, there are multiple bacterial species that reside on the eyelid, and each is capable of reacting to the population density of the other, making blepharitis a polymicrobial disease. That quorum sensing regulates behaviors integral to bacterial function makes it worthwhile to eavesdrop on their conversations in hopes of intercepting messages that may otherwise have led to pathology.
Blepharitis research can benefit from the study of other polymicrobial diseases. What might appear to be an insignificant bacterial disease may contribute to the onset of other diseases. For instance, periodontitis, a disease of the gum tissue caused by pathogenic oral microflora, has been associated with cardiovascular disease,9 diabetes and stroke.10,11 The University of Calgary's Michael Surette and his co-workers brought the lethal lung disease cystic fibrosis back to the lab to re-visit the pathogenic players in the lung infections that are ultimately fatal for CF patients. Pseudomonas aeruginosa is the main culprit, but the lung is also home to commensal bacteria that exist there without causing ill effects to the body. Dr. Surette's group found evidence that the commensal bacteria could be drawing the ire of P. aeruginosa via interspecies communication.8 This highlights the importance of considering all types of bacteria when researching the mechanism of polymicrobial diseases, and the practical consideration of optimizing culturing methods to detect any and all potential flora. The ideal clinical model would take into account the role of bacteria in blepharitis, but solid bacterial endpoints are still elusive.
Developing a Clinical Model
Improvements in clinical technology and diagnostics will move us in the direction of understanding and distinguishing the various manifestations of blepharitis, which will aid in the development of customizable treatments. Using close-range photography to examine the eyelid at an impressive level of detail, we have revealed that blepharitis is a focal disease. The blepharitic eyelid is not uniformly diseased; the disease state and/or the severity of the disease can differ among neighboring glands and sections of the eyelid. Therefore, an improved clinical model should focus on these diseased areas, which represent modifiable endpoints. Through identification of individual diseased glands, researchers can track and assess the same gland over the entire course of a clinical trial using standardized scales to grade meibum viscosity and clarity. Additionally, the severity of eyelid hyperemia can also be graded using a similar standardized scale.
The Search for Treatment
Ophthalmic researchers can learn much from the work of microbiologists. The inadequacies of simple treatments of eyelid scrubs and antibiotics to control the dynamic bacteria flora have been exposed. In the wake of increasing antibiotic resistance, research is already under way to determine if intercepting bacterial communications is a viable alternative to antibiotics. As Dr. Novick has shown in a model of abscess formation, this strategy proves promising.12 Unfortunately, current findings cannot be directly applied to the treatment of blepharitis because each bacterial disease has its own players and perhaps unique bacterial conversations.
Exposing normal flora to antibiotics that do not target the pathogenic population can lead to increased antibiotic resistance.13,14 Antibiotic treatment with the goal of reduction or alteration of pathogenic bacterial levels, rather than the eradication of all bacteria, may be more conducive to overall clinical improvement and ocular health. This phenomenon has been observed in other regions of the body: An imbalance of gastrointestinal tract flora can exacerbate inflammatory bowel disease and Crohn's disease. Treatments for these diseases are aimed at restoring balance, highlighting the importance of maintaining commensal bacteria at sites of infection.
As Lewis Carroll wrote, we must begin at the beginning. We have to characterize quorum sensing as it applies to blepharitis. The tools are available to gain a better understanding of the disease. With new, more sensitive photographic methods we can look more closely at the distinguishing clinical manifestations of blepharitis, which will help define study populations and inspire clinical endpoints. Also, with the realization of quorum sensing comes a more complete understanding of the contribution of bacteria to the disease process, and a promise of novel therapies. If nothing else, quorum sensing is a humble reminder to stop and smell the research every once in a while, so that we can offer new, more thorough and accurate therapies.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology 1982;89:10:1173-80.
2. Thygeson P. Etiology and treatment of blepharitis. Archives of Ophthalmology 1946;36:445-477.
3. Groden LR, Murphy B, Rodnite J, Genvert GI. Lid flora in blepharitis. Cornea 1991;10:1:50-3.
4. Kulacoglu DN, Ozbek A, Uslu H, Sahin F, Gullulu G, Kocer I, et al. Comparative Lid Flora in Anterior Blepharitis. Turk J Med Sci 2001;31:359-363.
5. Allansmith MR, Anderson RP, Butterworth M. The meaning of preoperative cultures in ophthalmology. Trans Am Acad Ophthalmol Otolaryngol 1969;73:4:683-90.
6. Schauder S, Bassler BL. The languages of bacteria. Genes Dev 2001;15:12:1468-80.
7. Nealson KH, Platt T,
8. Duan K, Dammel C, Stein J, Rabin H, Surette MG. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 2003;50:5:1477-91.
9. Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med 2004;15:6:403-13.
10. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:9499:1809-20.
11. Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: A randomised, placebo-controlled crossover trial. Lancet 2002;360:9338:978-84.
12. Wright JS, 3rd, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A 2005;102:5:1691-6.
13. Kowalski RP, Karenchak LM, Romanowski EG. Infectious disease: Changing antibiotic susceptibility. Ophthalmol Clin North Am 2003;16:1:1-9.
14. Smolin G, Okumoto M. Staphylococcal blepharitis. Arch Ophthalmol 1977;95:5:812-6.
Further
1. Federle MJ, Bassler BL. Interspecies communication in bacteria. J Clin Invest 2003;112:9:1291-9.
2. Bassler BL, Losick R. Bacterially speaking. Cell 2006;125:2:237-46.
3. Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 2003;48:6:1429-49.
4. Novick RP. Interrupters on the bacterial party line. Nat Chem Biol 2005;1:6:321-2.



