Retinal venous occlusive disease is the most common retinal vascular disorder after diabetic retinopathy. Early recognition and treatment are important to avoid potentially significant visual morbidity. Depending on the location of venous blockage, retinal vein occlusion (RVO) is classified as branch retinal vein occlusion (BRVO), central retinal vein occlusion (CRVO), or hemi-retinal vein occlusion (HRVO). This article reviews the pathogenesis, natural history and management of each of these forms of RVO.
Branch Retinal Vein Occlusion
Branch retinal vein occlusion, which typically occurs at an arteriovenous (AV) intersection, is the most common RVO. The artery and vein share a common adventitial sheath at the AV crossing with varying degrees of fusion of the vascular wall. Venous compression by the relatively rigid artery may result in turbulent flow, endothelial damage, thrombosis and occlusion. In normal eyes, the artery is anterior to the vein in roughly two-thirds of the intersections, while this number rises to almost 100 percent at the site of obstruction in a BRVO.1 Most BRVOs (63 percent) occur in the supertemporal quadrant, due to the increased number of arteriovenous crossings in this quadrant. Because of their asymptomatic nature, nasal occlusions are less likely to be diagnosed and are probably underreported.
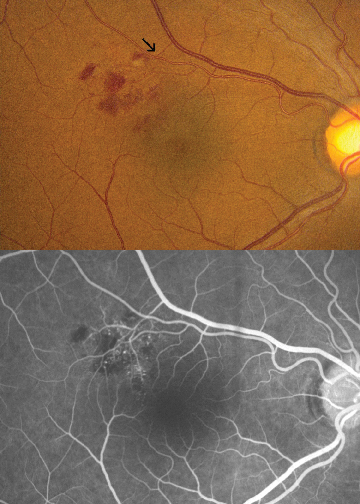 |
| Figure 1. Small macular branch retinal vein occlusion showing compression of vein by overlying sclerotic artery (arrow). Fluorescein angiography shows microvascular abnormalities within the distribution of the occluded vein. |
BRVO occurs at a mean age of 60 years. Epidemiological data from the Beaver Dam Eye Study suggest a prevalence and five-year incidence of 0.6 percent each.2 Many ocular and systemic conditions are associated with BRVO. The Eye Disease Case-Control Study identified systemic hypertension (associated with 50 percent of cases), cardiovascular disease, increased body mass index at age 20 years, a history of glaucoma, and high serum levels of alpha 2-globulin as risk factors for BRVO, while moderate alcohol consumption and higher HDL levels were associated with reduced risk of BRVO.3
Clinically, patients with BRVO may complain of decreased vision or may be asymptomatic. Vision loss at presentation is related to the extent of macular damage (See Figure 1) from intraretinal edema (most common), hemorrhage or capillary non-perfusion. In the acute phase, the involved retina typically shows venous dilation and tortuosity, superficial intraretinal hemorrhages, and/or cotton wool spots. Cystoid macular edema may be present, sometimes with blood in a foveal cyst. The characteristic fluorescein angiographic findings in BRVO include delayed venous filling in the area of occlusion, capillary non-perfusion, and macular edema in the acute phase, as well as microvascular abnormalities in later stages. More recently, serous macular detachment and subretinal hemorrhage have been documented using optical coherence tomography.4 Over time, the retinal edema and hemorrhage may resolve spontaneously with recovery of vision. Long-term sequelae include sheathing of veins, vascular telangiectasis, the formation of collateral retinal vessels, the development of retinal (See Figure 2) or disc neovascularization, vitreous hemorrhage, and traction retinal detachment.
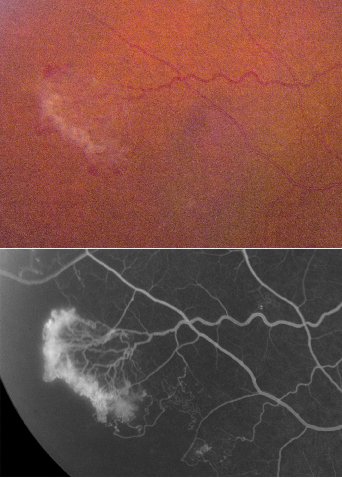 |
| Figure 2. Branch retinal vein occlusion with mid-peripheral retinal neovascularization at edge of the avascular zone. Fluorescein angiography shows capillary non-perfusion and leakage from the NV. |
Current management is based on the recommendations of the Branch Vein Occlusion Study (BVOS)5,6 and newer evolving surgical techniques. Laboratory evaluation is not recommended in a typical BRVO, while atypical cases such as BRVOs occurring in young patients, bilateral BRVOs, or a BRVO occurring at a site not associated with an arteriovenous crossing warrant a laboratory work up. In addition to ruling out known causes of vasculitis, such as Behçet's disease, sarcoidosis, syphilis and Lyme disease, tests to consider include serum homocysteine level, antinuclear antibody, lupus anticoagulant, and anticardiolipin antibody. The BVOS recommends an observation period of three months to allow for spontaneous improvement of macular edema and hemorrhage. If resolution does not occur, fluorescein angiography is used to assess the extent of macular edema, foveal hemorrhage, and perifoveal capillary drop out. Eyes with visual acuity of 20/40 or worse, persistent macular edema, absence of foveal hemorrhage and an intact perifoveal capillary network should undergo argon laser grid photocoagulation to the area of macular capillary leakage. In the BVOS, 65 percent of treated eyes improved at least two lines of visual acuity compared to 35 percent of the untreated eyes (mean follow up of 3.1 years). For eyes with macular ischemia only, no treatment is recommended.
The risk of retinal and disc neovascularization is proportional to the area of retinal ischemia. In the BVOS, retinal neovascularization developed in 22 percent of eyes with BRVO irrespective of the degree of non-perfusion, and in 36 percent of eyes with 5 or more disc areas of non-perfusion. Iris neovascularization developed in only 1.6 percent of eyes. The BVOS demonstrated that sector panretinal photocoagulation reduced the risk of retinal neovascularization from 22 percent to 12 percent (average follow up 3.7 years), and it reduced the risk of vitreous hemorrhage in eyes with neovascularization from 60 percent to 30 percent (average follow up 2.8 years). However, the study also showed that in nonperfused eyes, 12 percent of eyes treated before the onset of neovascularization developed vitreous hemorrhage, and 9 percent of eyes treated after the onset of neovascularization developed vitreous hemorrhage. These data suggest that there may be no advantage to treating prior to the development of neovascularization, and therefore, the BVOS recommends that nonperfused eyes with BRVO should be treated only if neovascularization develops, or if the patient is unable to follow up on a regular basis.
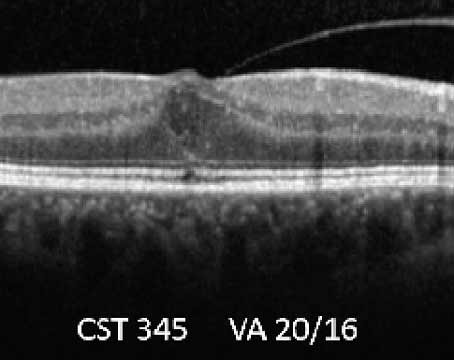
Off-label use of intravitreous triamcinolone acetonide is being increasingly used for the treatment of macular edema unresponsive to laser. Two or four milligrams (0.05 or 0.1 ml, respectively) of triamcinolone acetonide (Kenalog, Bristol-Myers Squibb) is injected through the inferior pars plana under sterile conditions in the outpatient clinic. Serial OCT is used as a rapid and less invasive way of monitoring the macular edema (See Figure 3a). Improvement in visual acuity and macular edema is probably secondary to stabilization of the blood-retinal barrier. Potential complications include infectious,7 sterile, or pseudo-endophthalmitis, elevated intraocular pressure, cataract formation, retinal detachment, and intraocular hemorrhage. Recurrence of retinal edema occurs commonly, often requiring repeated injections.
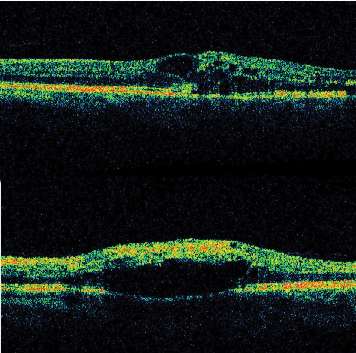 |
| Figure 3. OCT demonstrating a) perifoveal intraretinal cystic changes in a patient with branch retinal vein occlusion, and b) macular intraretinal edema in a patient with central retinal vein occlusion. |
Surgical intervention (vitrectomy) in BRVO is indicated for non-clearing vitreous hemorrhage, for traction retinal detachment involving the macula, and for removal of epiretinal membrane. More recently, vitrectomy with separation of the posterior hyaloid with or without internal limiting membrane (ILM) peeling or intraocular gas tamponade has been advocated for the treatment of macular edema.8,9 This technique has been shown to improve visual acuity and reduce macular edema in a small series of patients. Early surgical intervention seems to be a critical factor for obtaining visual improvement. The exact mechanism of improvement is unclear, but may involve relief of vitreous or ILM traction, anterior egress of retinal edema fluid, increased oxygen supply to the macula, and/or tamponade of the macula by intraocular gas.
Another surgical technique being promoted is vitrectomy and AV sheathotomy to separate the artery and vein at the AV crossing.10 A number of non-randomized studies have shown that AV sheathotomy may be associated with improvement in visual acuity, macular edema, macular hemorrhage, and even retinal perfusion. Theoretically, relieving the pressure on the retinal vein from the relatively rigid arterial wall may restore venous flow, presumably following recanalization of the thrombus. Early intervention could be critical for a favorable outcome. It is unclear whether the beneficial effect is a function of the AV sheathotomy or the accompanying vitrectomy and posterior hyaloid separation. Limitations include technical difficulty in separating the vessels, and potential complications include hemorrhage, retinal detachment, and visual field defects.
Limitations of these newer interventional techniques include small sample size and lack of randomization. Prospective, randomized, controlled, multicenter clinical trials are required to accurately assess these results.
Central Retinal Vein Occlusion
Central retinal vein occlusion is a potentially blinding disorder characterized by the presence of intraretinal hemorrhages and edema in all four retinal quadrants. The occlusion typically occurs at or near the lamina cribrosa, where relative narrowing of the central retinal artery and vein contributes to turbulent flow and an increased risk of thrombosis, especially if the artery is sclerotic and rigid.
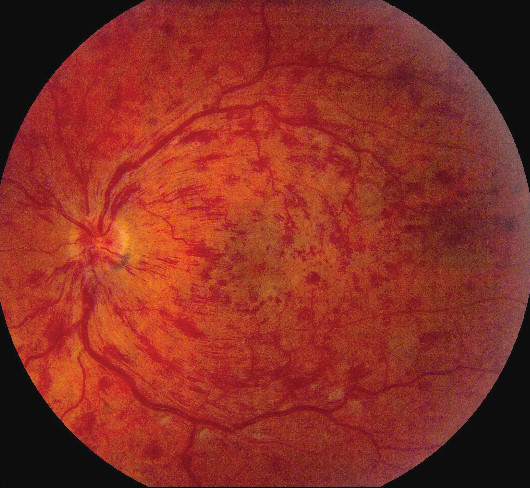 |
| Figure 4. Central retinal vein occlusion showing dilation and tortuosity as well as intraretinal hemorrhages in all four quadrants. |
The majority of patients with CRVO are older than 50 years and men are affected slightly more often than women. Epidemiological data from the Beaver Dam Eye Study suggest a prevalence of 0.1 percent and a five-year incidence of 0.2 percent.2 Younger patients are more likely to have an inflammatory etiology and a more benign course. The Eye Disease Case-Control Study identified systemic hypertension, diabetes mellitus, and open-angle glaucoma as risk factors for CRVO, while alcohol consumption and increased physical activity were associated with a reduced risk of CRVO.11 Additionally, oral contraceptive use, diuretics, hypercoagulable states, hyperviscosity, and vasculitis as might occur in Behçet's disease, sarcoidosis, syphilis, or Lyme disease have been associated with CRVO. Tests for thrombophilia may include activated protein C resistance, protein C activity, protein S activity, antithrombin III, homocysteine, lupus anticoagulant, and anticardiolipin antibody. In younger patients, an increased prevalence of collagen vascular disease has been reported. Bilateral CRVO has been associated with hyperviscosity syndromes such as primary and secondary polycythemia, leukemia/lymphoma, multiple myeloma, Waldenstrom macroglobulinemia, and cryoglobulinemia.
CRVO is classified into two distinct entities—ischemic and non-ischemic—each with differing clinical presentation, prognosis, complications and management options. An intermediate form also exists, but 80 percent of these intermediate cases progress to the ischemic variety over time.
Clinically, patients with CRVO typically present with sudden loss of central vision. Transient obscurations of vision can also occur, but are less common. In the acute phase all four quadrants show venous dilation and tortuosity, superficial and deep intraretinal hemorrhages, and/or cotton wool spots (See Figure 4). There is usually disc edema and there may be macular edema and/or serous detachment (See Figure 3b). The characteristic fluorescein angiographic findings in CRVO include delayed venous filling and capillary non-perfusion which may be obscured by the retinal hemorrhages. If the retinal hemorrhages are primarily in the peripheral retina and predominantly blot shaped, carotid insufficiency should be ruled out. Although the severity of the ophthalmoscopic findings varies with the extent of retinal ischemia, ophthalmoscopy alone is not adequate to differentiate between ischemic and non-ischemic CRVO.
Clinically, visual acuity <20/200, the presence of an afferent papillary defect, and extensive retinal hemorrhages are consistent with ischemic CRVO. Abnormal ancillary test results such as an altered electroretinogram (reduced scotopic and photopic b-wave amplitude), widespread non-perfusion (>10 disc areas) on fluorescein angiography, and peripheral visual field defects (as assessed by Goldmann perimetry) indicate ischemic CRVO. In some cases it may be difficult to assess the perfusion status by FA until the retinal hemorrhages resolve.
In CRVO, vision loss is primarily due to macular edema, macular hemorrhage, and perifoveal capillary non-perfusion in the ischemic variety. In eyes with ischemic CRVO, approximately 58 percent eventually develop iris neovascularization, 47 percent develop angle neovascularization, 33 percent develop neovascular glaucoma and approximately 8 percent develop retinal neovascularization.12 Ischemic CRVO runs a self limited course and eventually burns itself out. Nonischemic CRVO typically resolves after a variable period of time and tends not to cause ocular neovascularization, although some eyes may convert to ischemic status. Two-thirds of patients with non-ischemic CRVO retain a visual acuity of 20/40 or better without any treatment.
Current treatment is based on the central vein occlusion study (CVOS)13,14 recommendations. All patients should undergo close follow up (every three to four weeks) including undilated slit lamp examination of the iris and gonioscopy. Panretinal photocoagulation is recommended if and when iris or angle neovascularization is detected (the CVOS used two clock hours of NVI). Prophylactic panretinal photocoagulation for ischemic CRVO is recommended only if close follow-up is not possible. According to the CVOS, grid laser photocoagulation for macular edema results only in angiographic improvement but not visual improvement and therefore is not recommended.
Numerous surgical techniques with varying degrees of success are being advocated for the treatment of CRVO. These include administration of tissue plasminogen activator (t-PA), laser-induced chorioretinal anastomosis, radial optic neurotomy, and intravitreous triamcinolone acetonide for macular edema.
Currently, there is no definitive data to support the use of systemic anticoagulation for prophylaxis or treatment of CRVO. Thrombolytic agents such as t-PA have been used via the systemic, intravitreous,15 and retinal venous route.16 Systemic administration is associated with the risk of systemic and intraocular bleeding. Intravitreous administration of t-PA shows outcomes that are similar to the natural history of CRVO. Cannulation and injection of t-PA into a peripapillary branch retinal vein specifically targets the thrombus at the lamina cribrosa, but it is not clear if the reported beneficial effects are from lysis of the thrombus or from mechanical dilation of the central retinal vein. Logically, this technique should be used in the acute stage before the thrombus is organized.
Laser induced anastomosis17 between a retinal vein and the choroid is a procedure that seeks to bypass the central retinal vein outflow. Argon laser is used to disrupt the wall of the vein as well as the underlying Bruch's membrane. Limitations of this technique include a low success rate (30 to 50 percent) and complications such as vitreous hemorrhage, choroidal neovascularization, preretinal fibrosis and traction retinal detachment.
Radial optic neurotomy18 is a procedure that attempts to decompress the central retinal vein at the lamina cribrosa. A radial incision of the lamina and sclera is made at the nasal edge of the optic disc. Concerns with this technique include damage to the nerve fiber layer, the arterial circle of Zinn, and the central retinal artery. In addition, it is not known if the reported beneficial effects are secondary to the incision or to the vitrectomy alone.
Intravitreous triamcinolone acetonide administration has been used to treat macular edema in both ischemic and non-ischemic CRVO.19 The technique and complications are similar to those described for BRVO. Reduction in macular thickness with improved visual acuity can last three to six months; however, improvement is unlikely in eyes with chronic macular edema and secondary macular changes. Furthermore, especially in the ischemic group, visual acuity may not improve despite resolution of macular edema.
Several new multicenter, prospective, randomized, controlled clinical trials are in progress or being planned for evaluating new pharmacologic treatments for RVO with macular edema. The Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) Study is an NIH-funded Phase III trial designed to assess the efficacy and safety of standard care versus triamcinolone acetonide injection(s) for the treatment of macular edema associated with CRVO and BRVO. The Intravitreous Steroid Injection Study (ISIS) is also evaluating intravitreous injections of triamcinolone acetonide for macular edema related to RVO. Eyetech Pharmaceuticals is conducting a Phase II clinical trial designed to test the efficacy and safety of intravitreous pegaptanib sodium injection (Macugen) for the treatment of macular edema secondary to CRVO. Allergan is sponsoring a study evaluating the Posurdex implant, a biodegradable extended-release device that delivers dexamethasone, for macular edema following CRVO and BRVO.
As with BRVO, most of these newer techniques and therapies need more extensive trials before they can be routinely performed. In addition, the time interval between the occlusive event and these interventions could be critical, and the duration of this window of opportunity is currently unknown.
Hemi-Retinal Vein Occlusion:
Hemi-retinal vein occlusions are variants of central retinal vein occlusions that involve the superior or inferior half of the retina. This pattern develops due to an anatomic variation at the optic nerve head. In approximately 20 percent of eyes, the veins draining the inferior and superior halves of the retina merge posterior to the lamina cribrosa, such that one of these two veins may be spared by the occlusive process resulting in a HRVO. The pathophysiology of HRVO mimics CRVO, but the clinical findings and complications are intermediate between CRVO and BRVO. For most clinicians, macular edema following HRVO is managed similar to that following BRVO, while CRVO guidelines are used for panretinal photocoagulation.
Dr. Buddi is a fellow in the Retina Service and Dr. Eliott, the director of the Retina Service and an associate professor of ophthalmology at the Kresge Eye Institute, Wayne State University. Contact Dr. Eliott at (313) 993-0871; fax: 313 577-2905; e-mail: deliott@med.wayne.edu.
1. Duker JS, Brown GC. Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol 1989;107(7):998-1000.
2. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc 2000;98:133-41.
3. The Eye Disease Case-Control Study Group. Risk factors for branch retinal vein occlusion. Am J Ophthalmol 1993;116(3):286-96.
4. Spaide RF, Lee JK, Klancnik JK Jr, Gross NE. Optical coherence tomography of branch retinal vein occlusion. Retina 2003;23(3):343-7.
5. Branch Vein Occlusion Study Group: Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol 1984;98(3):271-82.
6. Branch Vein Occlusion Study Group: Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Arch Ophthalmol 1986;104(1):34-41.
7. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 2003;136(5):791-6.
8. Mandelcorn MS, Nrusimhadevara RK. Internal limiting membrane peeling for decompression of macular edema in retinal vein occlusion. A report of 14 cases. Retina 2004;24(3):348-355.
9. Saika S, Tanaka T, Miyamoto T, Ohnishi Y. Surgical posterior vitreous detachment combined with gas/air tamponade for treating macular edema associated with branch retinal vein occlusion: retinal tomography and visual outcome. Graefes Arch Clin Exp Ophthalmol 2001; 239(10):729-32.
10. Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol 1988;106(10):1469-71.
11. The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol 1996;114(5):545-54.
12. Hayreh SS, Rojas P, Podhajsky P, et al. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology 1983;90(5):488-506.
13. The Central Vein Occlusion Study Group N report. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. Ophthalmology 1995;102(10):1434-44.
14. The Central Vein Occlusion Study Group M report. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology 1995;102(10):1425-33.
15. Weizer JS, Fekrat S. Intravitreal tissue plasminogen activator for the treatment of central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 2003;34(4):350-2.
16. Weiss JN, Bynoe LA. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology 2001;108(12):2249-57.
17. McAllister IL, Constable IJ. Laser-induced chorioretinal venous anastomosis for treatment of nonischemic central retinal vein occlusion. Arch Ophthalmol 1995;113(4):456-62.
18. Opremcak EM, Bruce RA, Lomeo MD, et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina 2001;21(5):408-15.
19. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol 2002;86(2):247-8.